39 octahedral crystal field splitting diagram
CHEM2P32 Lecture 11. Square and Tetrahedral Complexes The tetrahedral crystal field stabilization energy is calculated the same way as the octahedral crystal field stabilization energy. The orbital splitting diagram for square planar coordination can thus be derived from the octahedral diagram. As ligands move away along the z-axis, d-orbitals with a... Figure 1. Crystal field splitting for the octahedral coordinated Co... field split Co 3d states on the octahedral and tetrahedral cobalt sites. ... ... Ab initio studies suggest there are challenges associated with fabricating a quality p-type Co 3 O 4 material with fewer defects and high mobility, and these properties are essential to enhancing the performance of the all-oxide...
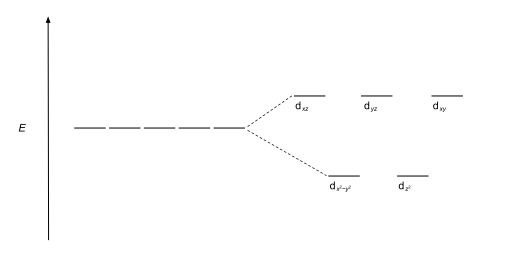
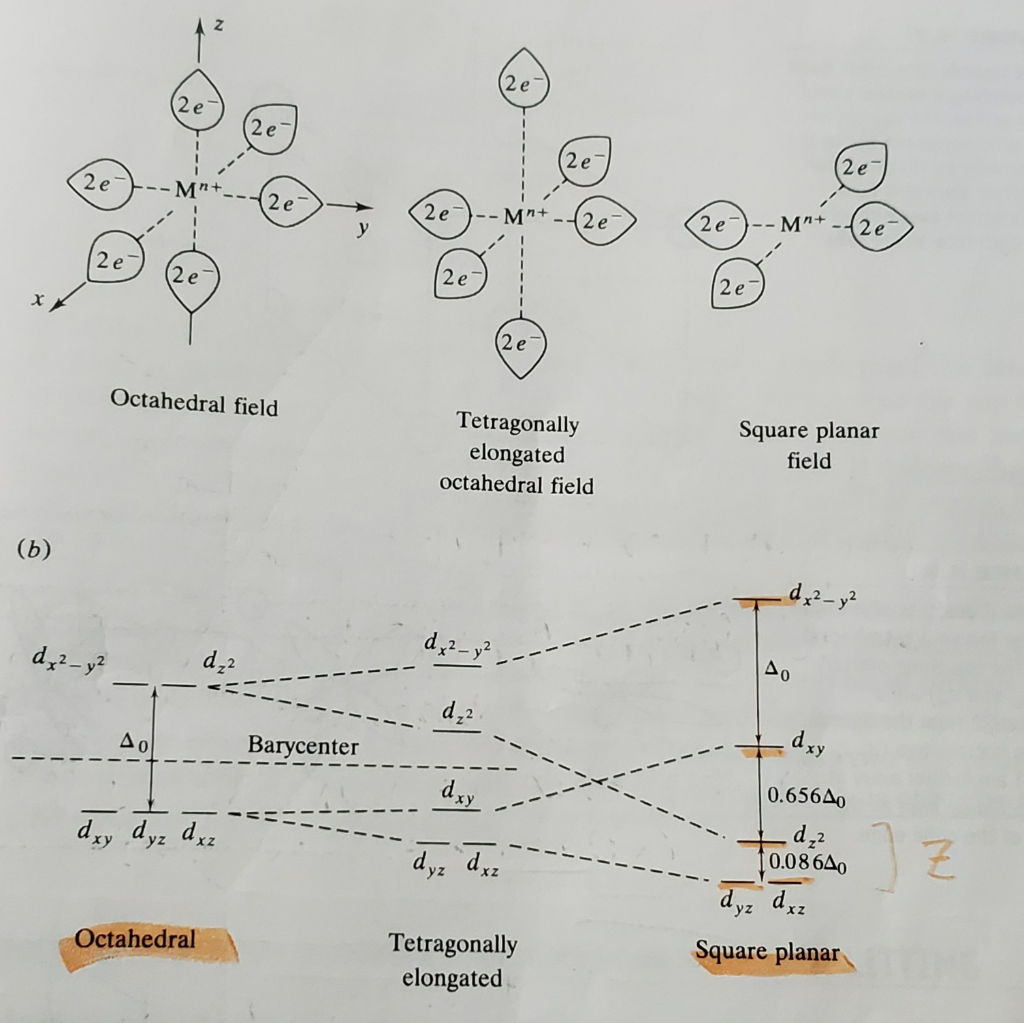
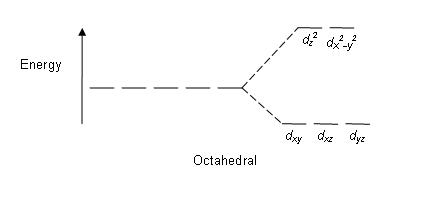
Crystal Field Splitting-Octahedral, Tetrahedral and Square Planar Crystal field for octahedral complexes. In an octahedral complex, there are six ligands attached to the central transition metal. The d-orbital splits into two different levels. The bottom three energy levels are named dxy ,dyz ,dxz (collectively referred to as t2g ).

Octahedral crystal field splitting diagram
PDF (d) Estimate the octahedral crystal field splitting energy... The weak field case has four unpaired electrons and the strong field has zero unpaired electrons. High Spin. Briefly explain your answer. (d) Estimate the octahedral crystal field splitting energy (Δo) in joules/mol if the wavelength most intensely absorbed is 740nm. What is the crystal field splitting of an octahedral complex? - Quora Crystal field splitting for octahedral complex is: So, because of above reason in dx2-y2 and dz2 there is repulsion between the orbitals and ligands and it goes higher in You can calculate crystal field splitting energy by using above diagram for various complexes with six number of ligands present. crystal-field splitting energy | Britannica Figure 18: Crystal field splitting. In an octahedral complex, the d orbitals of the central metal ion divide into two sets of different energies. The separation in energy is the crystal field splitting energy, Δ. (A) When Δ is large, it is energetically more favourable for electrons to occupy the lower...
Octahedral crystal field splitting diagram. Lecture 9 - Crystal field theory for octahedral The resulting d-orbital splitting diagram for tetrahedral coordination is the inverse of the diagram for octahedral coordination, as shown below. Crystal Field Stabilization Energy in Square Planar Complexes. Square planar coordination is rare except for d8 metal ions. Among the d8 metal ions... Solved Draw the octahedral crystal field splitting diagram Chemistry questions and answers. Draw the octahedral crystal field splitting diagram for each metal ion. Cr3+ Cu2+ Mn3+ (high-spin) Mn3+ (low-spin) Fe2+ (low-spin). inorganic chemistry - What does the crystal field splitting diagram for... The most basic crystal field argument includes point-symmetric charges approaching the central metal in a way as the ligands would. It remains to be shown why your diagram shows the orbitals at the energy levels it does. Simply speaking, there are no ligands in $z$ direction so anything containing $z... Crystal Field Theory (CFT). That is, the exact opposite of the situation we just dealt with for the octahedral crystal field. The end result is a splitting pattern which is represented in the In this course we will only be concerned with diagrams for octahedral, tetrahedral and square planar complexes. One of the important aspects of...
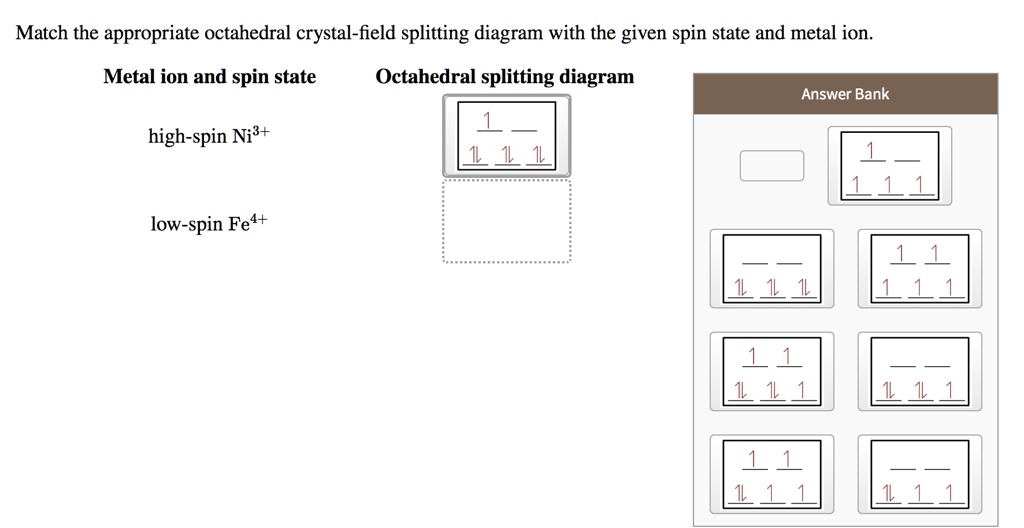
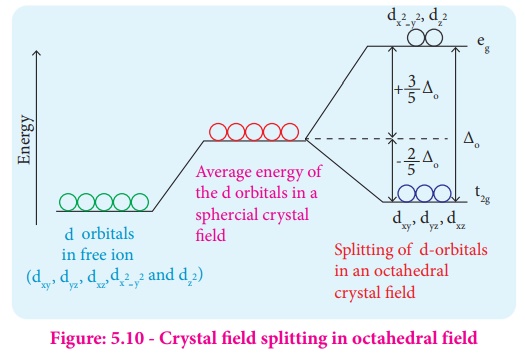
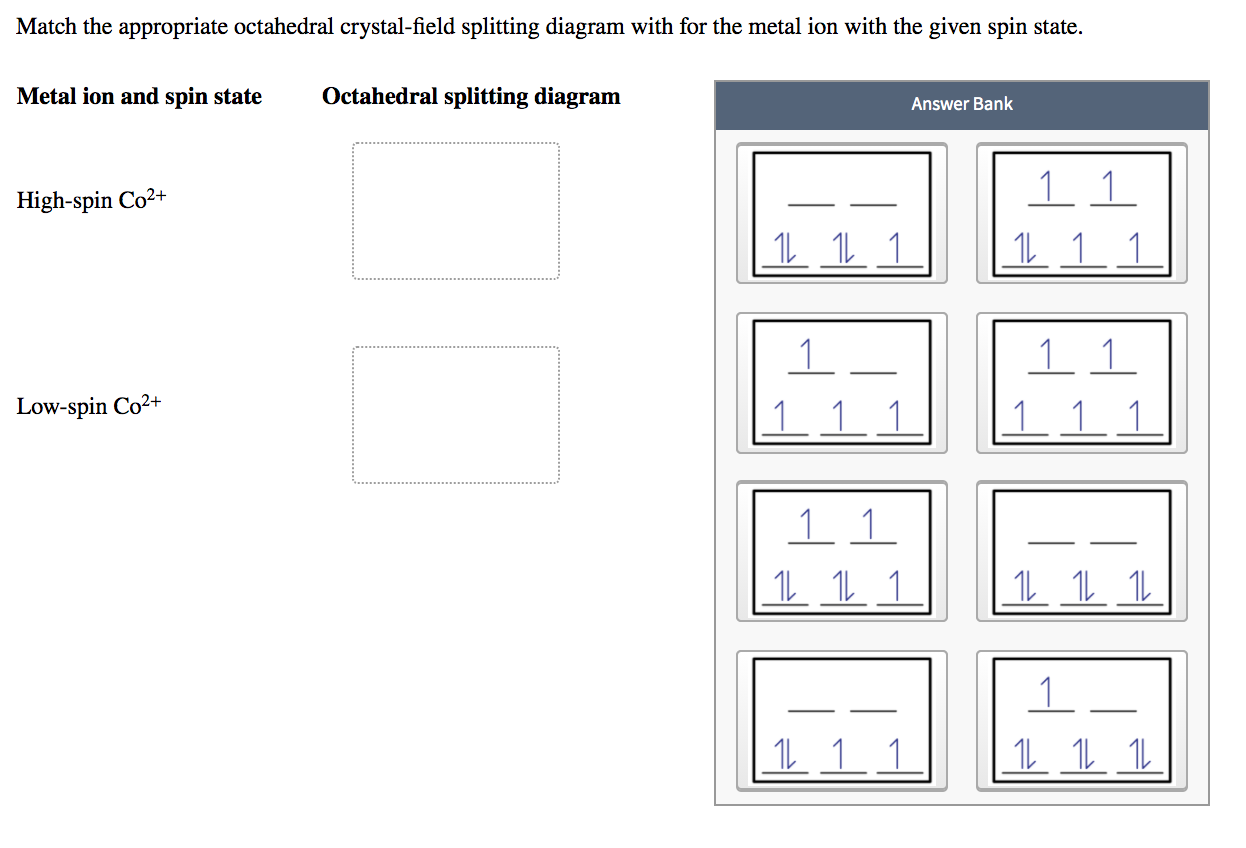
PDF Outline of crystal field theory | 3.4.2 Tanabe-Sugano Diagrams Crystal field splitting in octahedral coordination. The inverse fifth-power dependency of crystal field splitting on metal-oxygen distance expressed in eq. (2.17) is of fundamental importance in trans-ition metal geochemistry, particularly in mineral physics at high pressures and interpretations of visible... Octahedral Crystal Field Splitting - ppt download 1 Octahedral Crystal Field Splitting Why do d-orbitals split in these peculiar ways in presence of Octahedral ligand fields? The thick white line in the energy diagram rises slightly above the line marked "barycentre" on which it was initally resting OCTAHEDRAL CASE - dx2-y2 2 3 4 5. Match The Appropriate Octahedral Crystal Field Splitting Diagram Cobalt 3 has 6 d electrons cobalt normally has 9 valence electrons but youve lost 3. A none of the 3d orbitals point directly at ligands b... Crystal_field_theory | Crystal field splitting diagrams Crystal field theory Crystal field theory (CFT) is a model that describes the electronic structure of transition metal compounds, all of which can be. If the splitting of the d-orbitals in an octahedral field is Δoct, the three t2g orbitals are stabilized relative to the barycenter by 2/5 Δoct, and the eg...
File:Octahedral splitting diagram.svg - Wikimedia Commons File:Octahedral splitting diagram.svg. From Wikimedia Commons, the free media repository. Jump to navigation Jump to search. DescriptionOctahedral splitting diagram.svg. English: Crystal field splitting pattern of atomic d-orbitals for an octahedral metal complex. Crystal field theory - Wikipedia Crystal field theory (CFT) describes the breaking of degeneracies of electron orbital states, usually d or f orbitals, due to a static electric field produced by a surrounding charge distribution (anion neighbors). Crystal Field Splitting - an overview | ScienceDirect Topics Crystal field d orbital splitting diagrams for common geometries. The above treatment considers the ligands in an octahedral geometry (i.e., with the ligands placed at the centre of the faces of the cube). The square planar case is simply a special case of the octahedral symmetry where two ligands are... Match The Appropriate Octahedral Crystal Field Splitting Diagram Fe4 Crystal field stabilization energies for octahedral complexes four coordinate geometries crystal field theory ffqppor tetrahedral and squar...
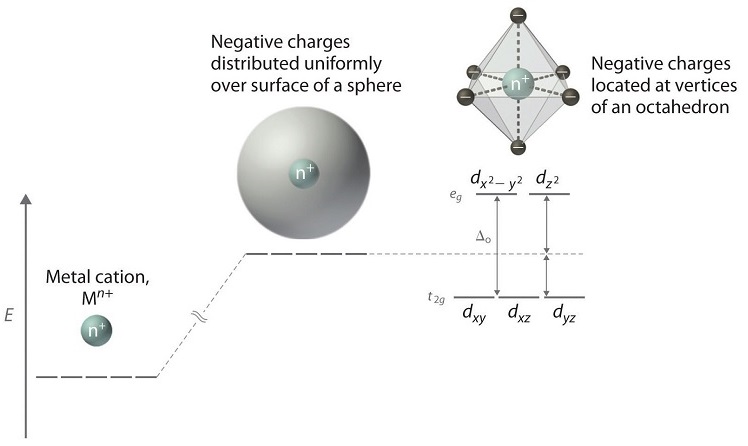
To study the crystal field splitting in Inorganic complexes. The splitting between these two orbitals is called crystal field splitting. The magnitude of stabilization will be 0.4 Δo and the magnitude of destabilization will The magnitude of the splitting of the t2g and eg orbitals changes from one octahedral complex to another. It depends on the identity of the metal...
SOLVED:Draw the octahedral crystal field splitting diagram for... So the octahedron from crystal field splitting takes place like this two off. So this system octahedron crystal field splitting a gram for Sierra Tripolis, then for See You two plus if he find out Directory confirmation of C two plus WE'LL see that See two plus has a different configuration.
Explain the following terms: (i) Crystal field splitting in an octahedral... The degenerate set of d-orbitals get split into two sets: the lower energy t2g set and eg set. The energy separation is denoted by ∆o . The actual configuration adopted is decided by the relative values of ∆o and P. P-represents the energy required for electron pairing in a single orbital. (a) If ∆o < P then fourth...
Crystal Field Theory | Octahedral Crystal Fields The tetrahedral crystal field splits these orbitals into the same t2g and eg sets of orbitals as does the octahedral crystal field. Octahedral transition-metal ions with d1, d2, or d3 configurations can therefore be described by the following diagrams. When we try to add a fourth electron, we are faced...
Table 23.10 Crystal Field Splitting Energies for Some Octahedral... Crystal field splitting does not change the total energy of the d orbitals. We can use the d-orbital energy-level diagram in Figure 23.10 "An Octahedral Arrangement of Six Negative Crystal Field Stabilization Energies. Recall from Chapter 9 "Molecular Geometry and Covalent Bonding Models"...
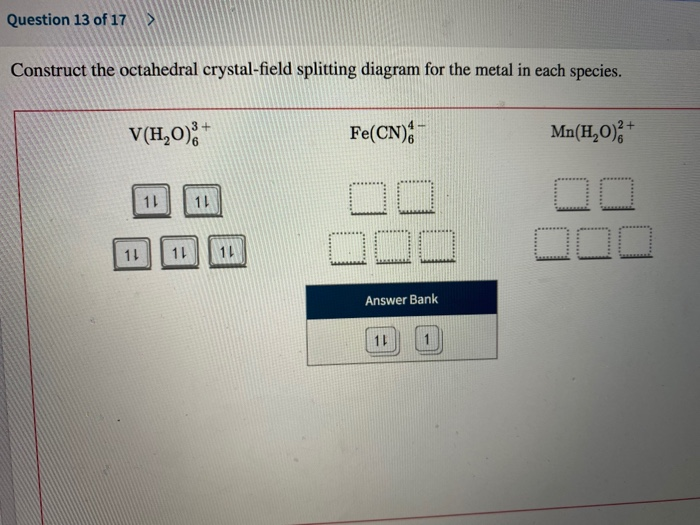
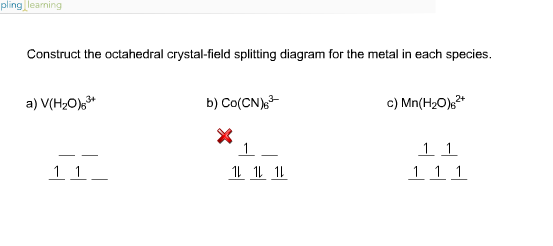
Construct the octahedral crystal-field splitting diagram for the metal... A: Every nuclei will have spin and if an external magnetic field is applied, there will be an energy tr... Q: For the following pair of reaction diagrams, identify which of the pair is catalyzed: 50 45 40 35 45... A: A catalysed reaction is the one in which a catalyst is used to increase the rate of reaction.
1s2p RIXS Calculations for 3d Transition Metal Ions in Octahedral... The calculations are performed in octahedral (Oh) symmetry using the crystal field multiplet theory. The diagrams of the relative energies with respect to the cubic crystal field, often without spin-orbit The crystal field splitting in Oh symmetry of the atomic terms of the initial state configuration of Ni2+...
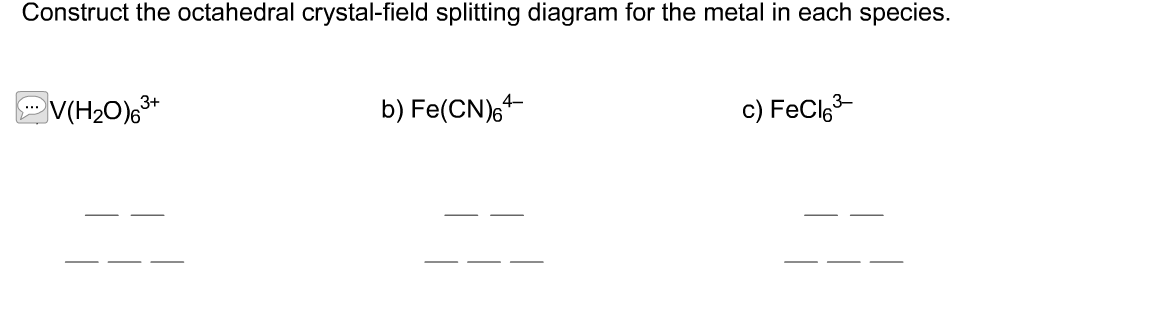
[Solved] Construct the octahedral crystal-field splitting diagram for... Q: Please help me with this question, thanks. Construct the octahedral crystal-field splitting diagram for the metal in eac. Q: Please Help Read these directions carefully!
PDF INTRODUCTION Then complexes' crystal field splitting parameters are calculated from the transition band with the Also another method, involving Tanabe-Sugano diagrams, was used to calculate crystal field Fig.1: Splitting of energy levels of a octahedral complex source. The degree of splitting of the d orbitals...
Octahedral CFT splitting: Electron diagram for octahedral d shell... Therefore, the crystal field splitting diagram for tetrahedral complexes is the opposite of an octahedral diagram. The dx2−dy2 and dz2 orbitals should be equally low in energy because they exist between the ligand axis, allowing them to experience little repulsion.
Crystal Field Theory - Chemistry LibreTexts | Octahedral Complexes Crystal field theory (CFT) describes the breaking of orbital degeneracy in transition metal complexes due to the presence of ligands. Placing a charge of −1 at each vertex of an octahedron causes the d orbitals to split into two groups with different energies: the dx2−y2 and dz2 orbitals increase in energy...
crystal-field splitting energy | Britannica Figure 18: Crystal field splitting. In an octahedral complex, the d orbitals of the central metal ion divide into two sets of different energies. The separation in energy is the crystal field splitting energy, Δ. (A) When Δ is large, it is energetically more favourable for electrons to occupy the lower...
What is the crystal field splitting of an octahedral complex? - Quora Crystal field splitting for octahedral complex is: So, because of above reason in dx2-y2 and dz2 there is repulsion between the orbitals and ligands and it goes higher in You can calculate crystal field splitting energy by using above diagram for various complexes with six number of ligands present.
PDF (d) Estimate the octahedral crystal field splitting energy... The weak field case has four unpaired electrons and the strong field has zero unpaired electrons. High Spin. Briefly explain your answer. (d) Estimate the octahedral crystal field splitting energy (Δo) in joules/mol if the wavelength most intensely absorbed is 740nm.





















0 Response to "39 octahedral crystal field splitting diagram"
Post a Comment