36 fluorescence energy level diagram
Molecular Expressions Microscopy Primer: Light and Color... Molecular Explanation of Fluorescence. Fluorescence activity is sometimes depicted diagrammatically as shown in Figure 4(a) (termed a Jablonski energy diagram). Explore how an electron absorbs energy and transcends to a higher energy state according to a Jablonski energy-level diagram. Raman and Fluorescence Spectroscopy of... Theoretical energy levels for Er, Nd, and Yb were used for the interpretation of fluorescence emission. The experimental results showed good agreement Additional fluorescence emission was observed with Yb2O3 that did not fit the reported energy level diagram. Tb4O7 was observed undergoing laser...
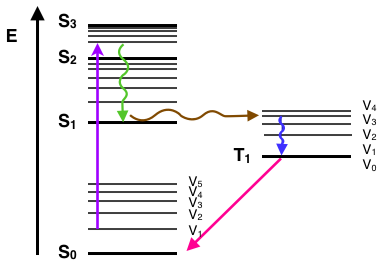
PDF Preview of "Jablonski diagram - ChemWiki" A Jablonski diagram is basically an energy diagram, arranged with energy on a vertical axis. The energy levels can be quantitatively denoted, but...The energy of the photon emitted in fluorescence is the same energy as the difference between the eigenstates of the transition;; however, the energy...
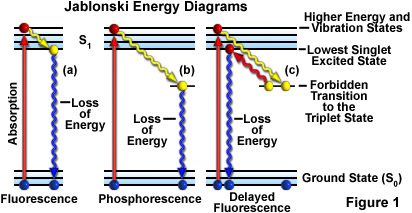
Fluorescence energy level diagram
PDF Slide 1 The Perrin-Jablonski diagram. Possible processes The energy spacing between the vibrational levels are almost similar in S0 and S1 so that the emission spectrum often appears Fluorescence: - involves the emission of light. - is the result of the absorption of light. - occurs after a finite duration... Production of X Rays | Radiology Key Atomic energy level diagram of tungsten with an electronic vacancy in the K shell that is filled by an electron from the M2 subshell. The two options: A as emission of characteristic (also called fluorescence) photon and B as emission of an Auger electron are described. Fluorescence - Wikipedia Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation. It is a form of luminescence. In most cases, the emitted light has a longer wavelength, and therefore a lower photon energy, than the absorbed radiation.
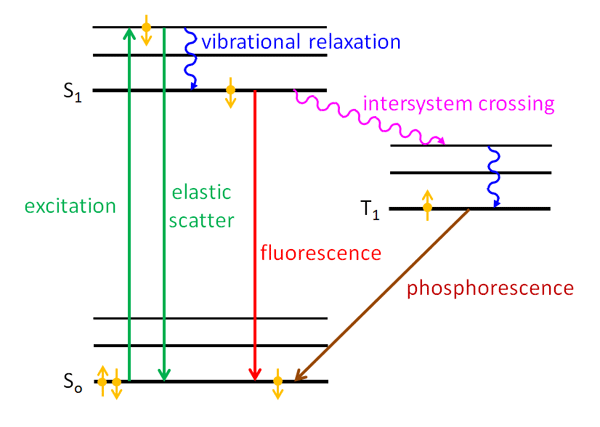
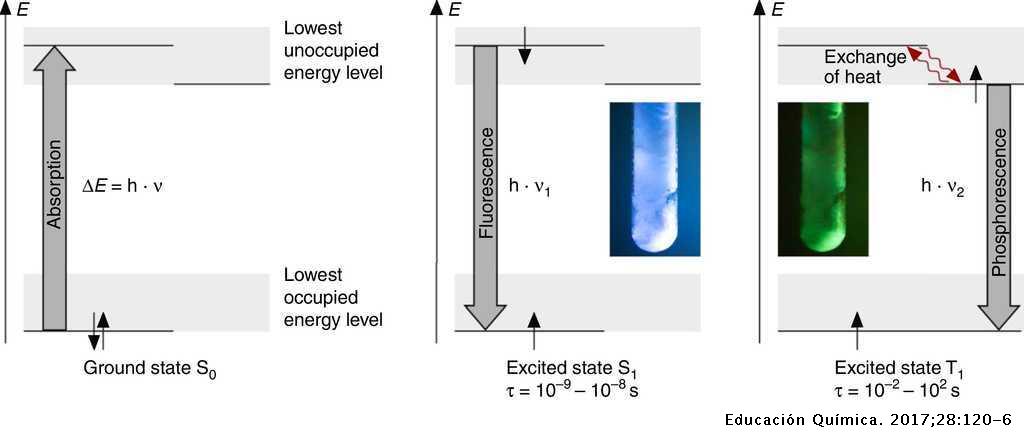
Fluorescence energy level diagram. Fluorescence | Encyclopedia.com Energy level —The internal energy state of an atom or molecule which is characterized by having only discrete, discontinuous values. In this case, the phenomenon is called phosphorescence. As an example of fluorescence, consider the energy level diagram for the gemstone ruby in Figure 1. PDF Slide 1 Energy of a Molecule (Jablonski energy-level diagram). Energy Levels for Luminescence Transitions. +quenching. Fluorescence in the Jablonski energy-level diagram. absorption fluorescence internal conversion intersystem crossing. S1. Fluorescent Dyes | Energy Level Diagram Observe the fluorescence from each beaker. Note that the fluorescent color of each solution is different than the color observed under the normal Explain how this relates to the "colorful glow" you see when a substance fluoresces. The glow is caused by the energy that is released by the electron... Principles of Fluorescence (With diagram) Return to Content. Principles of Fluorescence (With diagram). Article Shared by. When light is absorbed by a fluorochrome, its electrons become excited and move from a resting state (1) to a maximal energy level called the 'excited electronic singlet state' (2). The amount of energy required...
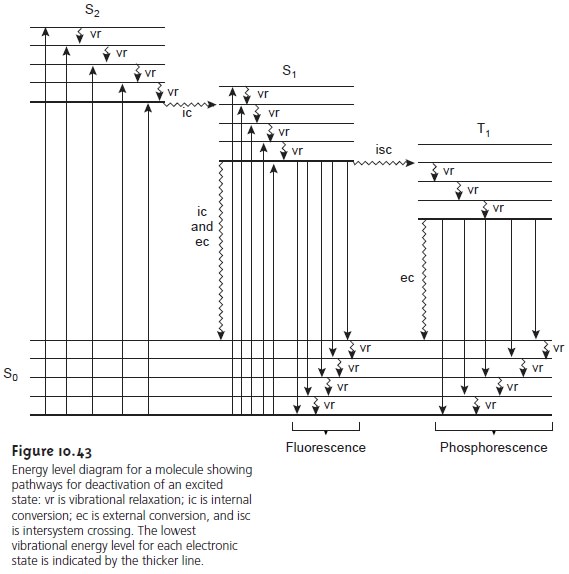
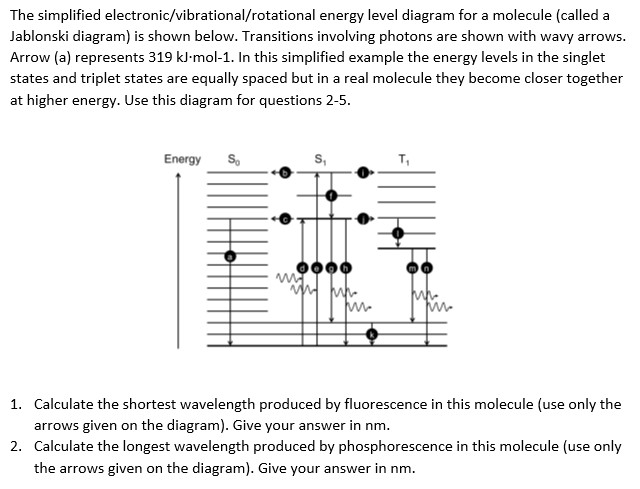
Frontiers | Energetics and Kinetics of S-State Transitions Monitored by... Understanding energetic and kinetic parameters of intermediates formed in the course of the reaction cycle (S-state cycle) of photosynthetic water oxidation is of high interest and could support the rationale designs of artificial systems for solar fuels. We use time-resolved measurements of the delayed... Draw a diagram of the energy levels for such a molecule. Fluorescence only occurs after a chemical species has first been excited by electromagnetic radiation. The emission of radiation by a solid object heated in There are also T2, T3, etc. as well. Now we can draw a more complex energy diagram for the molecule that shows different singlet and triplet levels... Fluorescence energy diagram - Big Chemical Encyclopedia Figure 12.1—Energy diagram comparing fluorescence and phosphorescence. Short arrows correspond to internal conversion without the emission of photons. Fluorescence is an energy transfer between states of the same multiplicity (spin state) while phosphorescence is between states of... PDF PowerPoint Presentation | Fluorescence yield vs. electron beam energy K-Fluorescence Photon Energy. • Secondary event: an electron from higher level fills out the K-shell vacancy (not all transitions are allowed by quantum mech. selection rules). • A fluorescence photon with the energy equal to the difference between the two energy levels may be emitted.
PDF Fig 4. Diagram of the energy levels of a fluorochrome molecule... Energy. S0. Fig 2. Jablonski diagram illustrating the processes involved in creating an excited electronic singlet state by optical absorption and subsequent Fluorescence results from a process that occurs when certain molecules (generally polyaromatic hydrocarbons or heterocycles) called... PDF Fluorescence However, the simple diagram above neglects the rotational levels associated with each vibrational level and which normally increase the number of possible From this level, the molecule can return to any of the vibrational levels of the ground state, emitting its energy in the form of fluorescence. PDF L4_Fluorescence.ppt Fluorescence. (Image: T. Wittman, Scripps). Edited by: Zvi Kam, Weizmann For Advance Light Microscopy course. OVERVIEW. • Principles of Fluorescence • Fluorophores • Fluorescence microscopes • Operational Considerations. Why uorescence? • High contrast. PDF It will be noted that benzenesulphonamide, which 1. Diagram relating energy levels, absorption, fluorescence, and phosphorescence. From these two equations, E = and, since h and state there is a loss of energy, for the energy level T. c are constants, E varies inversely as A. As mentioned above, the energy emitted as fluorescence is less...
Fluorescence Excitation & Emission | Emission Spectrums | Olympus LS Fluorescence Excitation and Emission Fundamentals. Fluorochromes are photoreactive chemical compounds that absorb light energy of a certain wavelength and Explore how an electron absorbs energy and transcends to a higher energy state according to a Jablonski energy-level diagram.
PDF Microsoft Word - 404 Fluorescen | Photon Emission (Fluorescence) Molecular Energy Levels and Absorption Spectra The phenomenon of fluorescence is intimately linked with that of absorption by molecules of energy in Vibrational Energy 'Sub-Level' Rotational Energy 'Sub-sub-level'. Internuclear Separation. We will use diagrams of this nature, which are...
Electronic Spectra of Molecules | Molecular Fluorescence The energy level diagrams contain much information about the molecule. The v=0 ground ground vibrational state has the highest probability at the The diagram above follows the suggestion of Beiser to explain how molecular fluorescence can occur. The electronic absorption shown follows the...
PDF Fluorescence and color | Figure 1 Energy level diagrams Fluorescence is a phenomenon that occurs when a substance absorbs radiation of a certain wavelength, or group of wavelengths, and re-emits photons of different wavelength. Figure 1 Energy level diagrams.
Figure 1. Jablonski energy diagram showing fluorescence and... Download scientific diagram | Jablonski energy diagram showing fluorescence and phosphorescence processes. The spot areas around hair follicles fluoresce red light, especially from 600 nm to 660 nm, matching well with prior fluorescence measurements of porphyrins [32] .
PDF Fluorescence Spectroscopy Fluorescence Spectroscopy. Yevgen Povrozin and Beniamino Barbieri. Published in Handbook of vibrational and rotational levels, which differ in energy by a smaller amount than the corresponding Figure 1. Perrin-Jablonsky energy diagram for a molecular structure. Singlet states are indicated by...
lecture 4 part 1 (fluorescence, Jablonski diagram) - YouTube lecture 4 part 1 (fluorescence, Jablonski diagram).
PDF Imaging | 1.1 Advantages of fluorescence detection Fig 4. Diagram of the energy levels of a fluorochrome molecule, including superimposed vibrational energy levels (a) and an example of 1.4 Quantitation of fluorescence. As discussed previously, the energy (wavelength) of the emitted fluorescent light is a statistical function of the available energy...
PDF Microsoft PowerPoint - Fluorescence [Compatibility Mode] 4. Energy level diagram (Jablonski diagram). Fluorescence process: Excitation. • At room temperature, everything starts out at the lowest vibrational energy level of the ground state.
PDF Fluorescence Resonance Energy Transfer Fluorescence Resonance Energy Transfer. Mathematical Modeling of FRET. Interaction of Electromagnetic Radiation with Metal Nanoparticles. 2.1 Energy level diagram describing A - the rotational changes, B - the vibrational changes and C - the electronic changes of the molecules...
Fluorescence Energy Transfer - an overview | ScienceDirect Topics Fluorescence energy transfer in particles is used, to achieve an excitation wavelength of 670 nm and an emission wavelength of 760 nm. At these wavelengths, scattering interferences and intrinsic absorbance and fluorescence from the plasma are minimized. The fluorescent dyes are...
Fluorescence - Wikipedia Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation. It is a form of luminescence. In most cases, the emitted light has a longer wavelength, and therefore a lower photon energy, than the absorbed radiation.
Production of X Rays | Radiology Key Atomic energy level diagram of tungsten with an electronic vacancy in the K shell that is filled by an electron from the M2 subshell. The two options: A as emission of characteristic (also called fluorescence) photon and B as emission of an Auger electron are described.
PDF Slide 1 The Perrin-Jablonski diagram. Possible processes The energy spacing between the vibrational levels are almost similar in S0 and S1 so that the emission spectrum often appears Fluorescence: - involves the emission of light. - is the result of the absorption of light. - occurs after a finite duration...

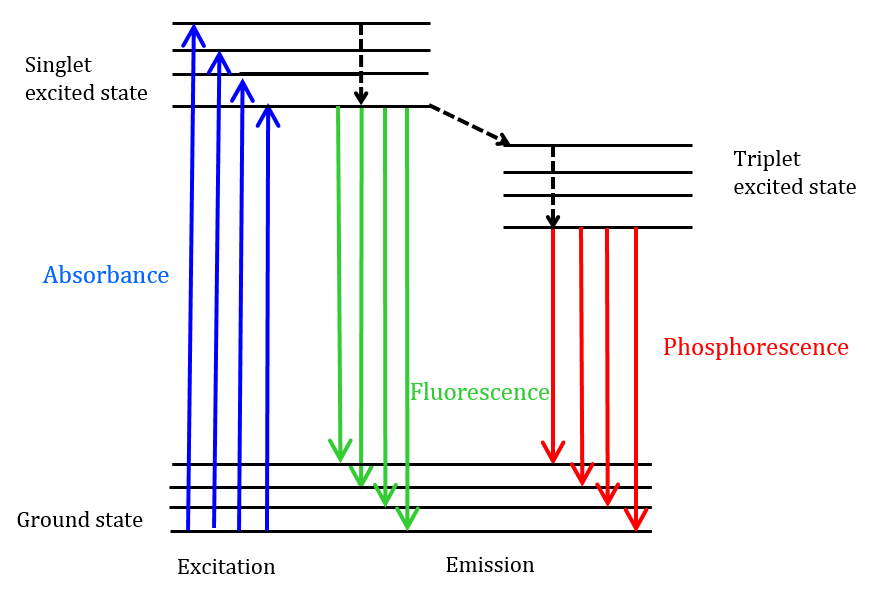


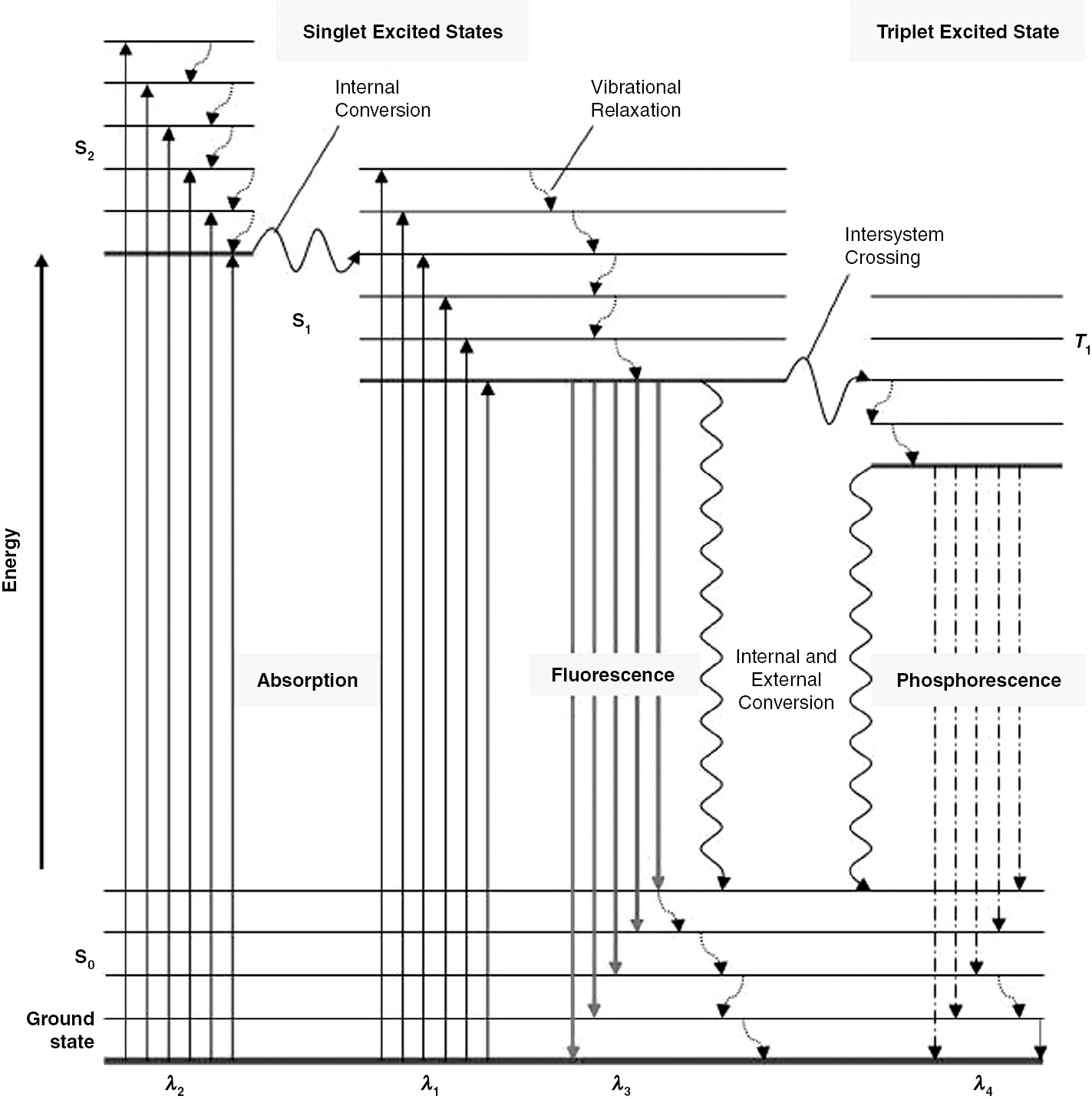


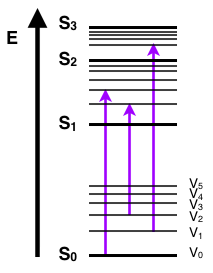








.png)


0 Response to "36 fluorescence energy level diagram"
Post a Comment