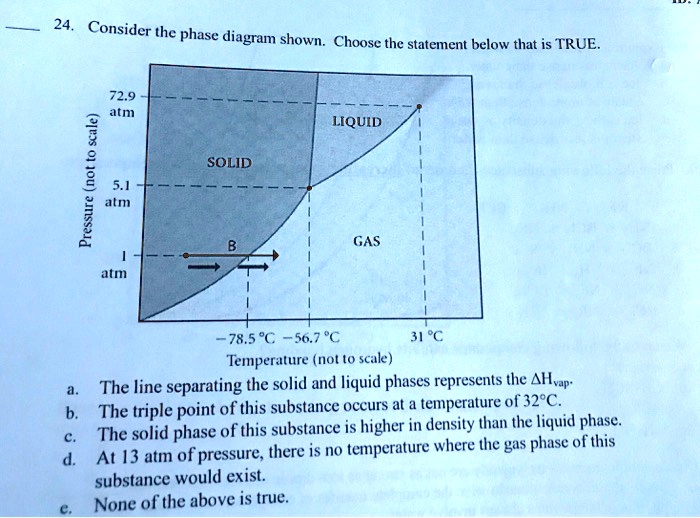
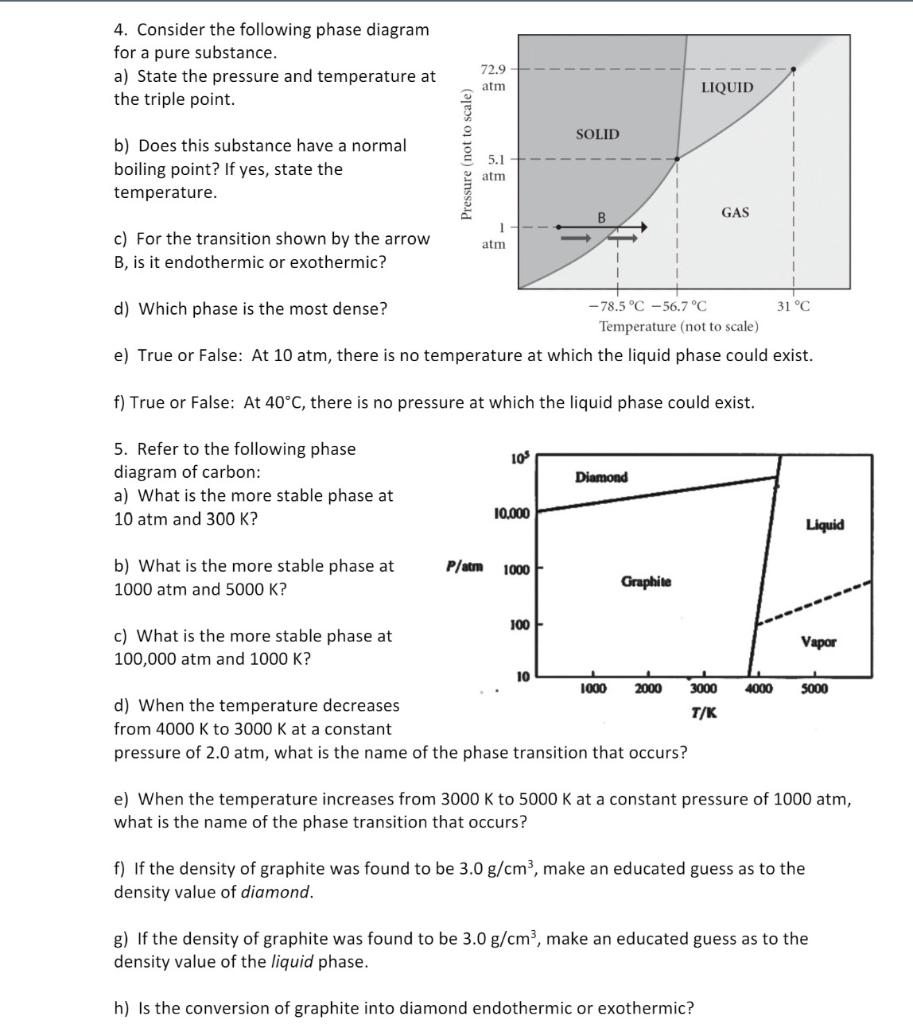
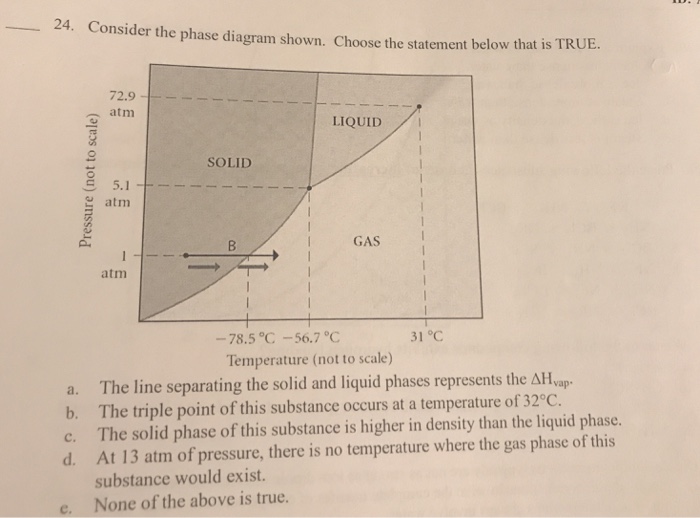
37 consider the phase diagram shown. choose the statement below that is true.
Choose the statement below that is TRUE. At 13 atm of pressure, there is no temperature where the solid phase of this substance would exist. The line separating the solid and liquid phases represents the ΔHvap. The triple point of this substance occurs at a; Question: Consider the phase diagram shown. Choose the statement below that is TRUE. Choose the statement below that is TRUE 2 atm LIQUID SOLID o am GASI atm -78.5 ?-56.7 °C 31°C Temperature (not to scale) a. The line separating the solid and ...
Part 1: Multiple Choice Questions (total 20 Points, ca. 20 minutes of work) Please check the appropriate box. For each question, there may be multiple right and wrong statements. Note that the number of points removed for an incorrect check is equal to the number of points granted for a correct check.
Consider the phase diagram shown. choose the statement below that is true.
Consider the phase diagram for carbon dioxide shown in Figure 5 as another example. The solid-liquid curve exhibits a positive slope, indicating that the melting point for CO 2 increases with pressure as it does for most substances (water being a notable exception as described previously). Notice that the triple point is well above 1 atm ... Quiz Flashcard. Sample quiz for Newton's laws of motion and free-body diagrams. Questions and Answers. 1. When analyzing dynamics problems, free-body diagrams. A. Should always be used. B. Are more useful when analyzing horizontal forces than when analyzing vertical forces. Consider the phase diagram for carbon dioxide shown in as another example. The solid-liquid curve exhibits a positive slope, indicating that the melting point for CO 2 increases with pressure as it does for most substances (water being a notable exception as described previously). Notice that the triple point is well above 1 atm, indicating ...
Consider the phase diagram shown. choose the statement below that is true.. Consider the phase diagram shown Choose the statement below that is TRUE LIQUID SO 1 GAS 78.5 %€ 56.7 "€ J"C Tatnpcruture (not jle) 0 The line ...4 answers · Top answer: a here is false. Ah, because that occurs at approximately 265 Kelvin. Part B is also false ... Choose the statement below that is TRUE. A) A solution will form between two substances if the solute-solvent interactions are of comparable strength to the solute-solute and solvent-solvent interactions. ... Assign the appropriate labels to the phase diagram shown below. A) A = liquid, B = solid, C = gas, D = critical point ... Consider the ... 9) 9) Consider the phase diagram shown. Choose the statement below that is TRUE 72.9 atm LIQUID SOLID atim GAS atm -78.5â -56.7â 31â Temperature (not to scale) A) The solid phase of this substance is higher in density than the liquid phase. Consider the phase diagram shown below. Choose the statement below that is TRUE. (A) The triple point of this substance occurs at a temperature of 72.9 atm. (B) At 10 atm of pressure, there is no temperature where the liquid phase of this substance would exist.
39. The diagrams above show four light bulbs of the same type. Two are in senes and two are in parallel. Which of the following statements are true? Choose two answers (A The light bulbs in the series circuit are brightest since they get the total current. e light bulbs in the pæallel circuit draw power than in the series circuit Indicate whether each of the following statements is true or false: (1) _____ The system is never an actor in a Use Case Diagram. (2) ... Consider the following UML Activity Diagram. ... Use Case Descriptions are more appropriate than Activity Diagrams for this purpose during the product design phase. (Hint: Think about how they are used and ... Transcribed image text: Consider the phase diagram shown. Choose the statement below that is TRUE. The solid phase of the substance is higher in density than the liquid phase. The line separating the solid and liquid phases represents the \ (\rm Delta H_ (vap)\). The triple point of this substance occurs at a temperature of 32 \ (\rm^ (\circ)C\). MS15a, Gibbs Free Energy and Phase Diagrams 11/00 . The system can, in fact, lower its free energy even further by splitting up into a solid of composition X. S B. and a liquid of composition X. L B (shown on both diagrams). The gibbs free energy of the solid is given by point (4) on the g(X. B) diagram and that of the liquid by point (5) on ...
Question 31. SURVEY. 120 seconds. Q. A straightedge and compass were used to creat the construction above. Arc EF was drawn from Point B, and arcs with equal radii were drawn from E and F. Choose all of the following that must be true. answer choices. We are given that a diagram . We have to find which statement are true about the given diagram. 1.CB is contained on the line n. CB is not contained on the line n because point C and B are not lying on the line n. Therefore, it not true. 2.CE is contained on the line m. CE is contained on the line m because point C and E are lying on the line m. most applications. In the phase diagrams we will discuss, pressure is assumed to be constant at one atmosphere (1 atm). The diagrams discussed here do not indicate the dynamics when one phase transforms into another. 9.6 Binary Isomorphous systems Consider the binary phase diagram for copper-nickel system, as shown. The abscissa ranges Phase Diagrams • Indicate phases as function of T, Co, and P. • For this course:-binary systems: just 2 components.-independent variables: T and Co (P = 1 atm is almost always used). • Phase Diagram for Cu-Ni system Adapted from Fig. 9.3(a), Callister 7e. (Fig. 9.3(a) is adapted from Phase Diagrams of Binary Nickel Alloys , P. Nash
Consider the phase diagram shown. Choose the statement below that is TRUE. A) The triple point of this substance occurs at a temperature of 31°C. B) At 10 atm of pressure, there is no temperature where the liquid phase of this substance would exist. C) The solid phase of this substance is higher in density than the liquid phase.
Consider the phase diagram shown. Choose the statement below that is TRUE. A) The triple point of this substance occurs at a temperature of 31°C. B) At 10 atm of pressure, there is no temperature where the liquid phase of this substance would exist. C) The solid phase of this substance is higher in density than the liquid phase.

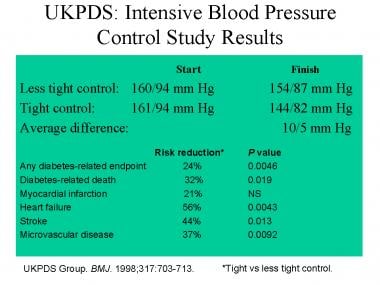
Kdigo 2020 Clinical Practice Guideline For Diabetes Management In Chronic Kidney Disease Kidney International
Consider the phase diagram shown. Choose the statement below that is TRUE. The solid phase of this substance is higher in density than the liquid phase.
Nov 18, 2019 — Get the detailed answer: 9) 9) Consider the phase diagram shown. Choose the statement below that is TRUE 72.9 atm LIQUID SOLID atim GAS atm ...
• A phase diagram of a pure compound has a triple point at 13 °C and 205 mmHg, a normal melting point at 17 °C, and a normal boiling point at 87 °C. Draw a phase diagram for this compound. Label all the different regions of the phase diagram. Marks 7 Indicate whether each of the following statements regarding this compound is true or false.
ANS: D PTS: 1 DIF: moderate REF: 11.3 OBJ: Define phase diagram. TOP: phases | phase transitions 31. Choose the correct statement about the diagram below. A) The diagram shows the triple point above 1 atm pressure. B) The diagram is qualitatively correct for water. C) The diagram shows that the melting point of the solid increases with ...
Solved 24 Consider The Phase Diagram Shown Choose The Statement Below That Is True 72 9 Atm 1 1 5 1 Am L Liquid Solid Gas Aum 78 5 56 7 31 Temperature Not
22) Consider the phase diagram shown. Choose the statement below that is true.A) The triple point of this substance occurs at a temperature of 31°C.B) At 10 ...
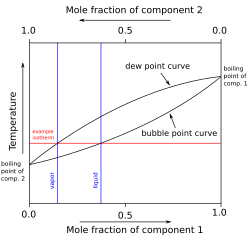
Phase Diagrams. The figure below shows an example of a phase diagram, which summarizes the effect of temperature and pressure on a substance in a closed container. Every point in this diagram represents a possible combination of temperature and pressure for the system. The diagram is divided into three areas, which represent the solid, liquid ...
Thus, the balanced voltages yield balanced currents. The phasor diagram for the line currents and the line-to-neu tral voltages is shown below. If we lay the line-to-neutral voltage phasors end to end, they form a closed triangle (the same property is true for the line currents). The closed triangle shows that the sum of these phasors is zero.
Consider the phase diagram shown. Choose the statement below that is TRUE. A) The triple point of this substance occurs at a temperature of 31°C. B) At 10 atm of pressure, there is no temperature where the liquid phase of this substance would exist. C) The solid phase of this substance is higher in density than the liquid phase.
Shown below is the Cu-Ni phase diagram (Figure 9.3a) and a vertical line constructed at a composition of 70 wt% Ni-30 wt% Cu. (a) Upon heating from 1300°C, the first liquid phase forms at the temperature at which this vertical line intersects the α-(α + L) phase boundary--i.e., about 1345°C. )-)-
21. Consider the phase diagram shown. Choose the statement below that is TRUE. A) The triple point of this substance occurs at a temperature of 31°C. B) At 10 atm of pressure, there is no temperature where the liquid phase of this substance would exist. C) The solid phase of this substance is higher in density than the liquid phase.
1. Refer to the phase diagram shown. The region enclosed by points DEF can be described as a (A) mixture of solid [3 component and liquid a component (B) mixture of solid f3 and liquid (3 component (C) peritectic composition (D) mixture of solid (3 component and a molten mix ture of a and (3 components
88) Assign the appropriate labels to the phase diagram shown below. B) A = gas, B = solid, C = liquid, D = triple point: 89) Consider the phase diagram shown. Choose the statement below that is TRUE. C) The solid phase of this substance is higher in density than the liquid phase. 90) Consider the phase diagram below.
Thank you! Select all statements that are TRUE, when considering the phase diagram shown. 10- atm B A 31 C -56 C Temperature (not to scale) Pressure ...1 answer · 0 votes: The last option is true. P Soid gas slople SH 2 Now; solid Ri equiibrumj From the Densin id Greater han SOlid Proeers , uwd Condensation Pis a roiple ...
4. Consider the phase diagram below. If the dashed line at 1 atm of pressure is followed from 100 to 500°C, what phase changes will occur (in order of increasing temperature)? A) condensation, followed by vaporization B) sublimation, followed by deposition C) vaporization, followed by deposition D) fusion (melting), followed by vaporization
Phasor diagrams provide a convenient graphical way of representing the quantities that change with time along with , which makes such diagrams useful for analyzing AC circuits with their inherent phase shifts between voltage and current.You have studied the behavior of an isolated resistor, inductor, and capacitor connected to an AC source.
Type 2 Diabetes Mellitus Treatment Management Approach Considerations Pharmacologic Therapy Management Of Glycemia
These diagrams indicate the physical states that exist under specific ... Consider the phase diagram for carbon dioxide shown in Figure 5 as another example ...
Consider the phase diagram for carbon dioxide shown in as another example. The solid-liquid curve exhibits a positive slope, indicating that the melting point for CO 2 increases with pressure as it does for most substances (water being a notable exception as described previously). Notice that the triple point is well above 1 atm, indicating ...
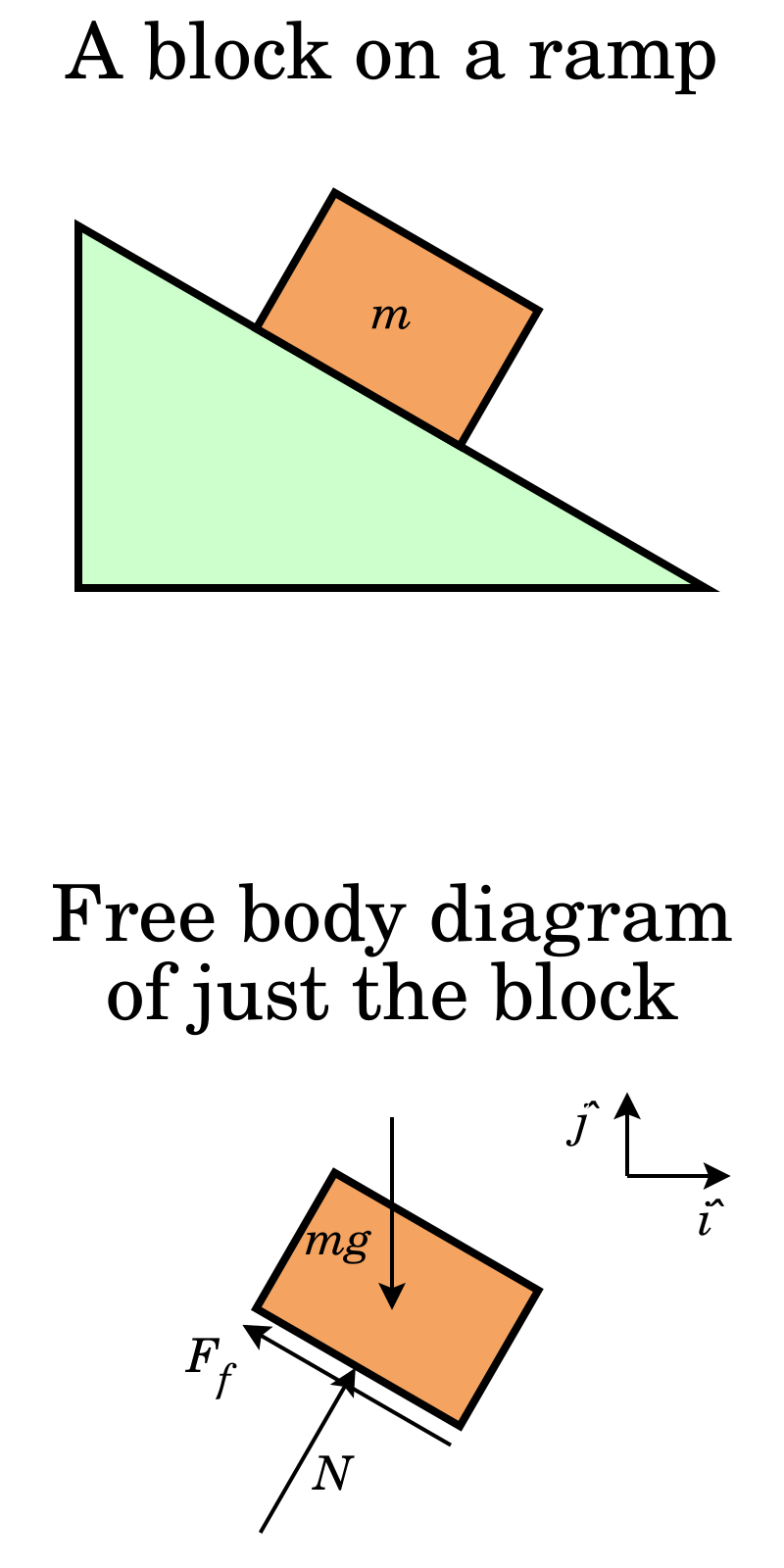
Quiz Flashcard. Sample quiz for Newton's laws of motion and free-body diagrams. Questions and Answers. 1. When analyzing dynamics problems, free-body diagrams. A. Should always be used. B. Are more useful when analyzing horizontal forces than when analyzing vertical forces.
Consider the phase diagram for carbon dioxide shown in Figure 5 as another example. The solid-liquid curve exhibits a positive slope, indicating that the melting point for CO 2 increases with pressure as it does for most substances (water being a notable exception as described previously). Notice that the triple point is well above 1 atm ...

Controlling Electrochemical Growth Of Metallic Zinc Electrodes Toward Affordable Rechargeable Energy Storage Systems
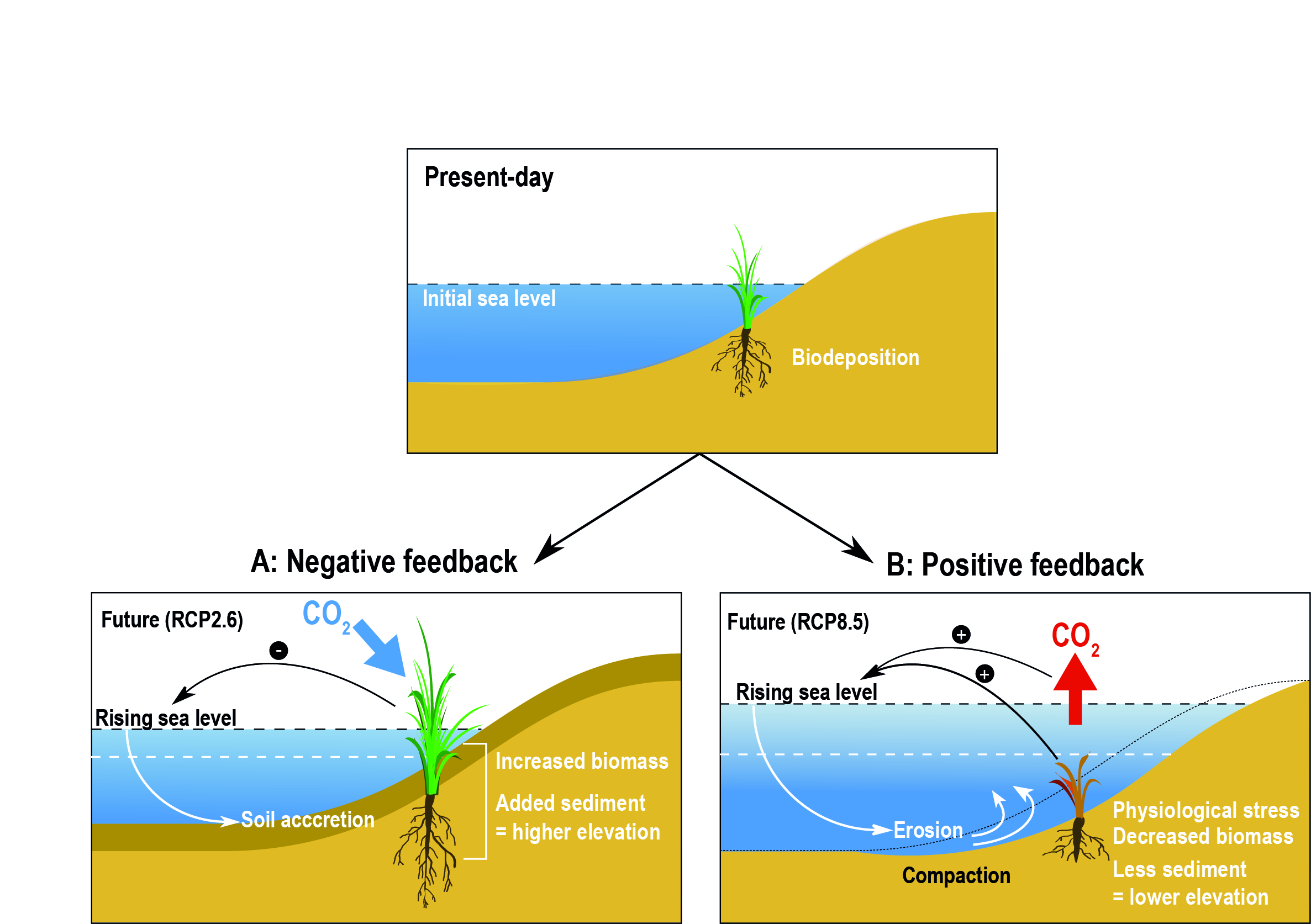
Chapter 5 Changing Ocean Marine Ecosystems And Dependent Communities Special Report On The Ocean And Cryosphere In A Changing Climate

Kdigo 2021 Clinical Practice Guideline For The Management Of Blood Pressure In Chronic Kidney Disease Kidney International




:max_bytes(150000):strip_icc()/dotdash_final_Graph2_Why_We_Splurge_When_Times_Are_Good_Feb_2020-1701a9a5d903401ba85edd21132b7f33.jpg)










0 Response to "37 consider the phase diagram shown. choose the statement below that is true."
Post a Comment