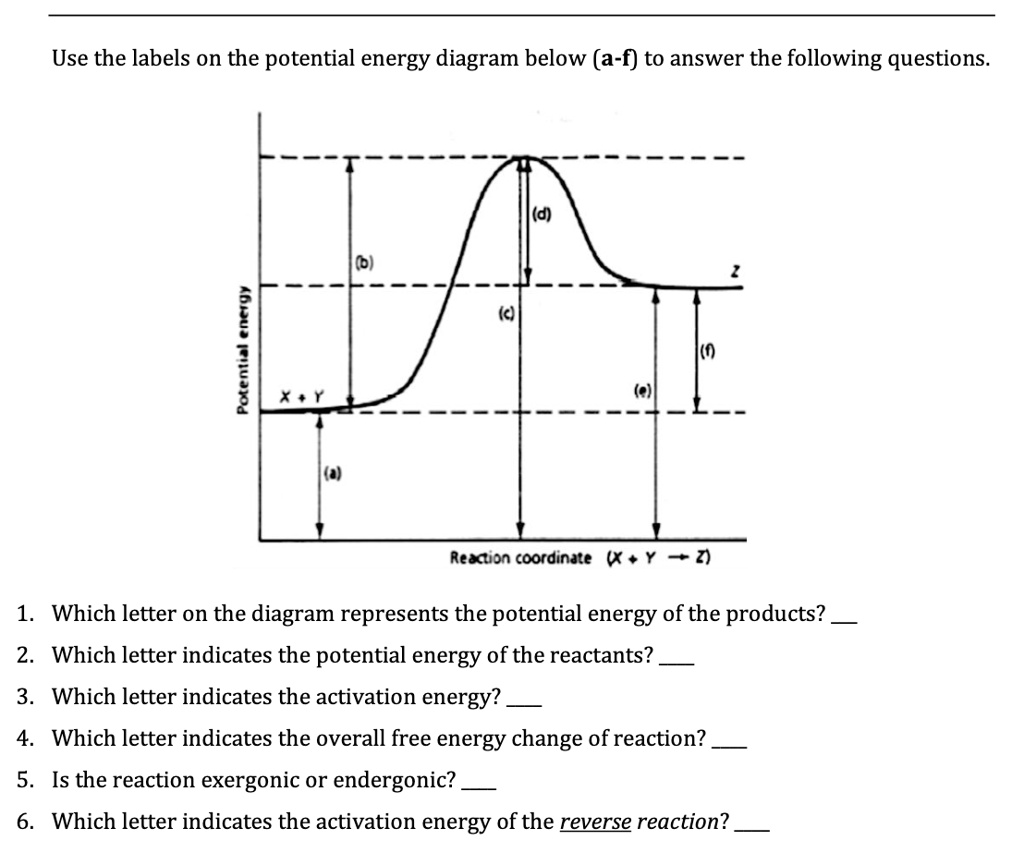
38 use the energy diagram for the reaction to answer the questions.
In the titration of 20.00 mL of 0.500 M CH3COOH by 0.500 M NaOH, calculate the pH of the solution at the point in the titration where 7.45 mL of 0.500 M NaOH has been added. Use the diagram to answer the questions below. Is the reaction endothermic or exothermic? What is the activation energy of the reaction? FREE Expert Solution. Recall that an energy diagram is usually read from left to right. The components of a one-step energy diagram are:
I am attempting to solve for the coefficient of rolling friction with the time being known for a cylinder rolling down a ramp. From my physics classes, I seem to remember that the two methods, being using a net force equation and using the conservation of energy principle, should give the same answer to a problem. However, the equations I have obtained using each of the two methods is different, which can be verified by plugging in the same time values in each. I am quite perplexed by this beca...

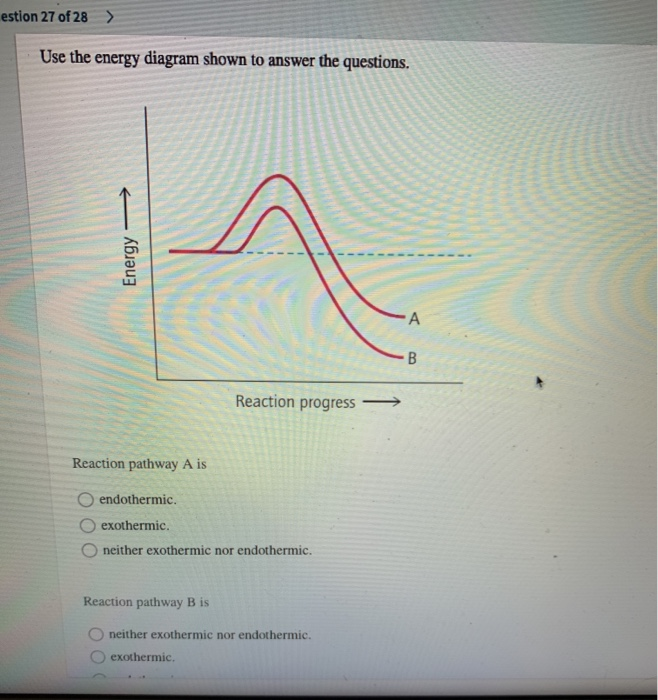
Use the energy diagram for the reaction to answer the questions.
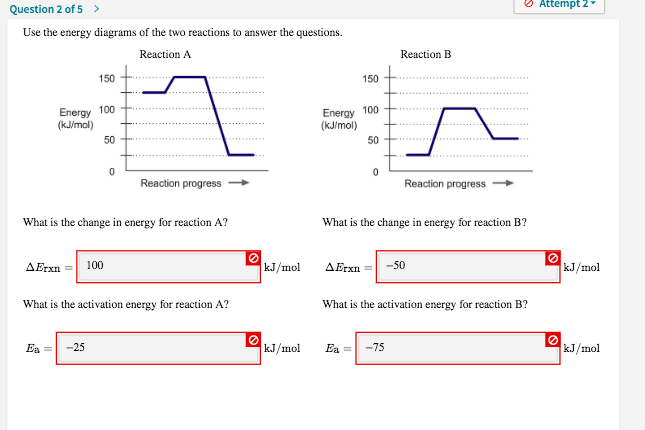
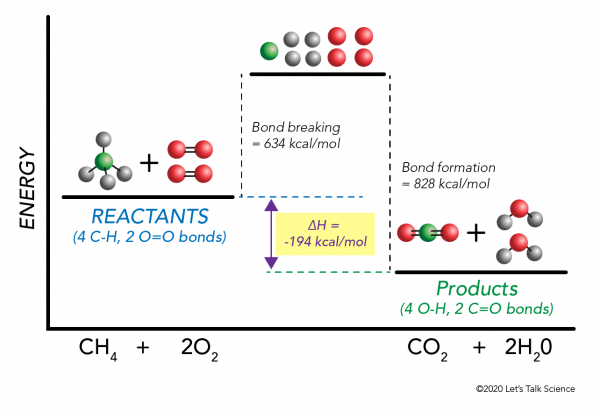
Hello there! I am on a bulk. I've never really found a proper answer to this, so I've come here now. When I do cardio, I make sure to eat the amount of calories that I've burned. Should I do the same when weightlifting, or is that the reason you eat in a surplus of +500 calories? ​ Cheers! Potential Energy Diagrams Worksheet CK-12 Foundation Chemistry Name Use the following Potential Energy Diagram to answer questions 1 - 12. 150 X2Y2 100 Potential Energy (kJ) 50 0 Progress of Reaction 1. Is the overall reaction as shown exothermic or endothermic? 2. What is the activation energy for the forward reaction? 3. What is the ... Question 15. SURVEY. 30 seconds. Q. How much energy is released when two moles of methane (CH 4) reacts at 298 K and 101.3 kPa according to the following equation: CH 4 (g) + 2O 2 (g) → CO2(g) + 2H2O (l) answer choices. 890.4 J. 890.4 kJ.
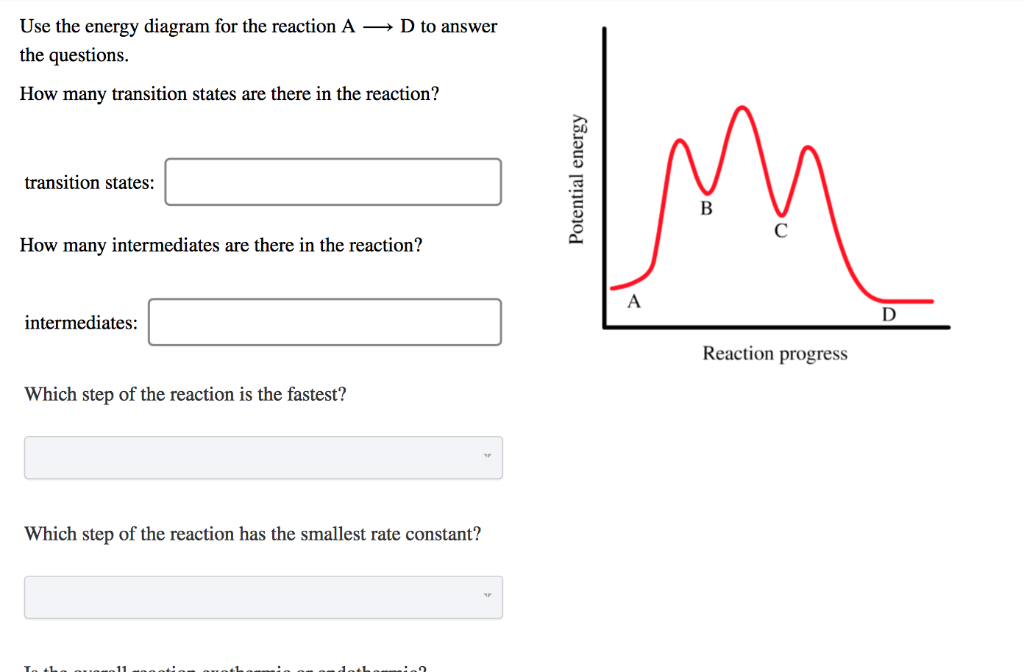
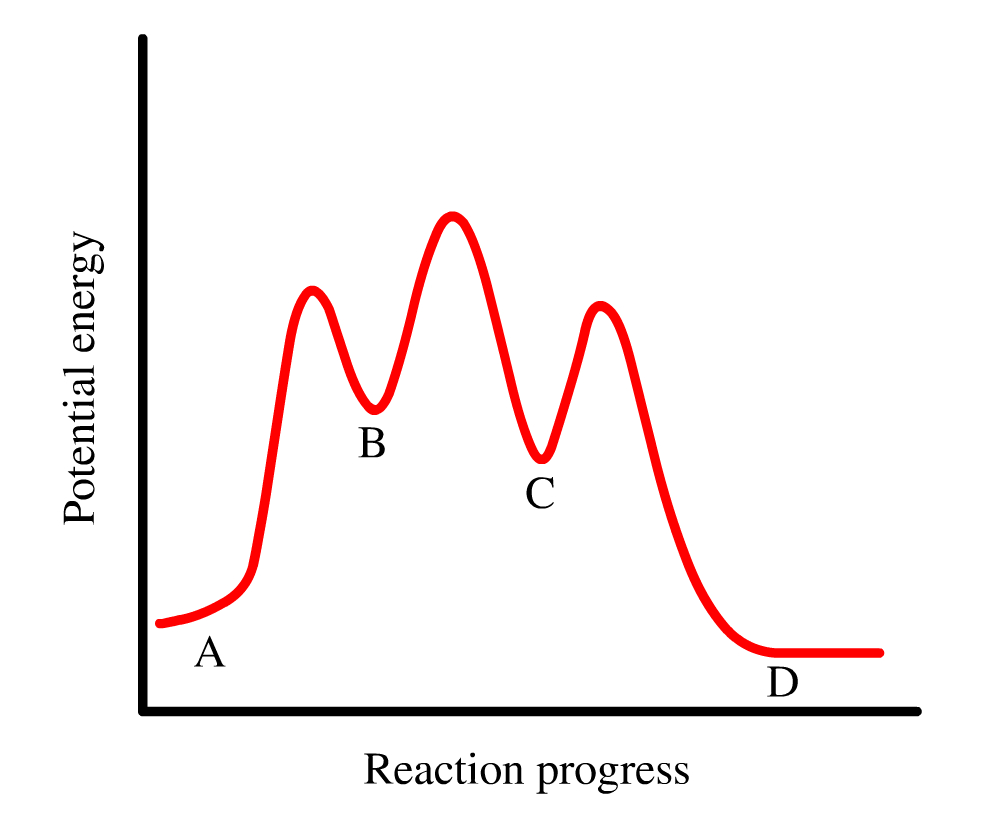
Use the energy diagram for the reaction to answer the questions.. USE THE POTENTIAL ENERGY DIAGRAM TO ANSWER THE QUESTIONS BELOW: 1. Is the overall reaction as shown . exothermic . or . endothermic ... the Activation Energy for the forward reaction and the Activation Energy for the reverse reaction on the graph above. 16. As reactant particles approach each other before a collision, the . Potential. Potential energy. Use the energy diagram for the reaction A → D to answer the questions. How many transition states are there in the reaction? transition states: B How many intermediates are there in the reaction? A D Reaction progress intermediates: Which step of the reaction is the fastest? Potential energy. Use the energy diagram for the rearrangement reaction of methyl isonitrile to acetoni- trile to answer the following questions. 7. What kind of reaction is represented by this diagram, endothermic or exothermic? 8. What is the chemical structure identified at the top of the curve on the diagram? Cor«plc¥ 9. What does the symbol E represent? 10. D to answer Use the energy diagram for the reaction A the questions. How many transition states are there in the reaction? transition states: Potential energy How many intermediates are there in the reaction? intermediates: Reaction progress Which step of the reaction is the fastest? Which step of the reaction has the smallest rate constant?
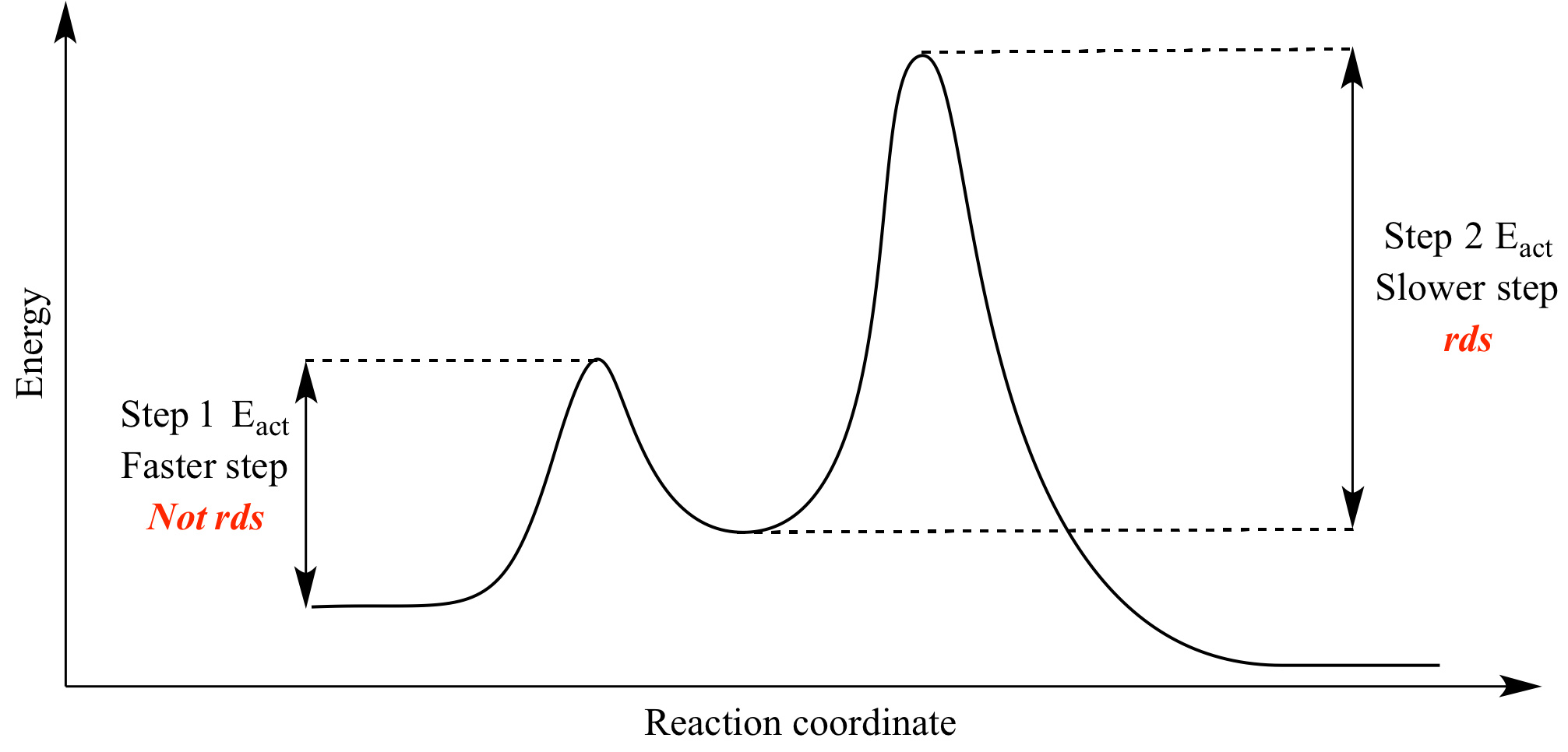
Use the energy diagram for the reaction A D to answer the questions. How many transition states are there in the reaction? How many intermediates are there in the reaction? Which step of the reaction is the fastest? Which step of the reaction has the smallest rate constant? Assume the frequency factor (?) is the same for each elementary reaction. We’re being asked if we can determine the activation energy of the reaction, given the energy diagram. Recall that the activation energy (E a) is the difference in energy between reactants (products for the reverse reaction) and transition state. E a for a reverse reaction is given by: E a = E transition state-E products. The equation given in the diagram is: A + B → C + D. The reverse of this reaction is: C + D → A + B Use the energy diagram for the reaction A D to answer the questions. How many transition states are there in the reaction? transition states: Potential energy How many intermediates are there in the reaction? intermediates: Reaction progress Which step of the reaction is the fastest? Which step of the reaction has the smallest rate constant? Chemistry*12* Potential*Energy*Diagrams*Worksheet* Name:* Date:* Block:*! USE!THE!POTENTIAL*ENERGY*DIAGRAM!TO!ANSWER!THEQUESTIONS!BELOW:! 1.! Is!the!overall!reaction ...
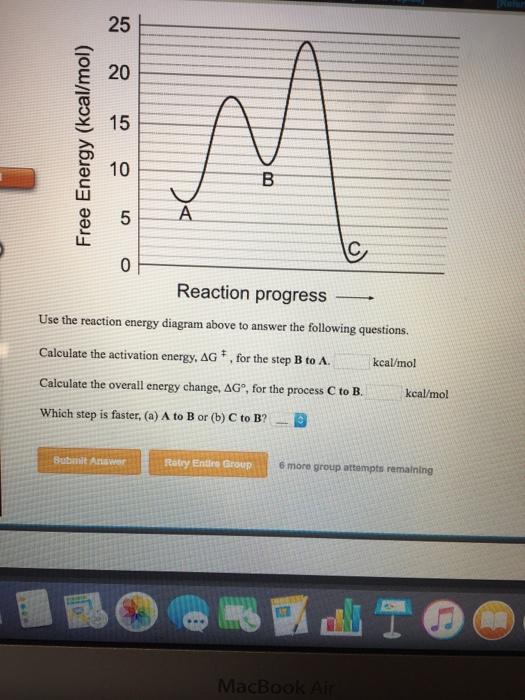
(b) €€€€The energy level diagram for this reaction is shown below. The energy changes, A, B and C, are shown on the diagram. Use the diagram to help you answer these questions. € (i) Which energy change, A, B or C, is the activation energy? (1) € (ii) Which energy change, A, B or C, shows that this reaction is exothermic? (1) Page 8 ... We’re being asked if we can determine the activation energy of the reaction, given the energy diagram.. Recall that the activation energy (E a) is the difference in energy between reactants (products for the reverse reaction) and transition state. E a for a reaction is given by:. E a = E transition state-E reactants. From the energy diagram, we can see that E reactants = 250 kJ and E ... Free Energy (kcal/mol) B. 25 20 15 10 Reaction progress Use the reaction energy diagram above to answer the following questions. Calculate the activation energy, AG, for the step B to C. kcal/mol Calculate the overall energy change, AG°, for the process A to B. kcal/mol Which step is faster, (a) C to B or (b) B to A? Free Energy (kcal/mol) B. These scammers can initially pretend not hearing you just so they can ask the question. Example: scammers may already have your credit card info and just use your voice to record your "yes" so they can authorize charges. It is real easy to spoof numbers so they might pose as a "legit" phone number to further their trickery.
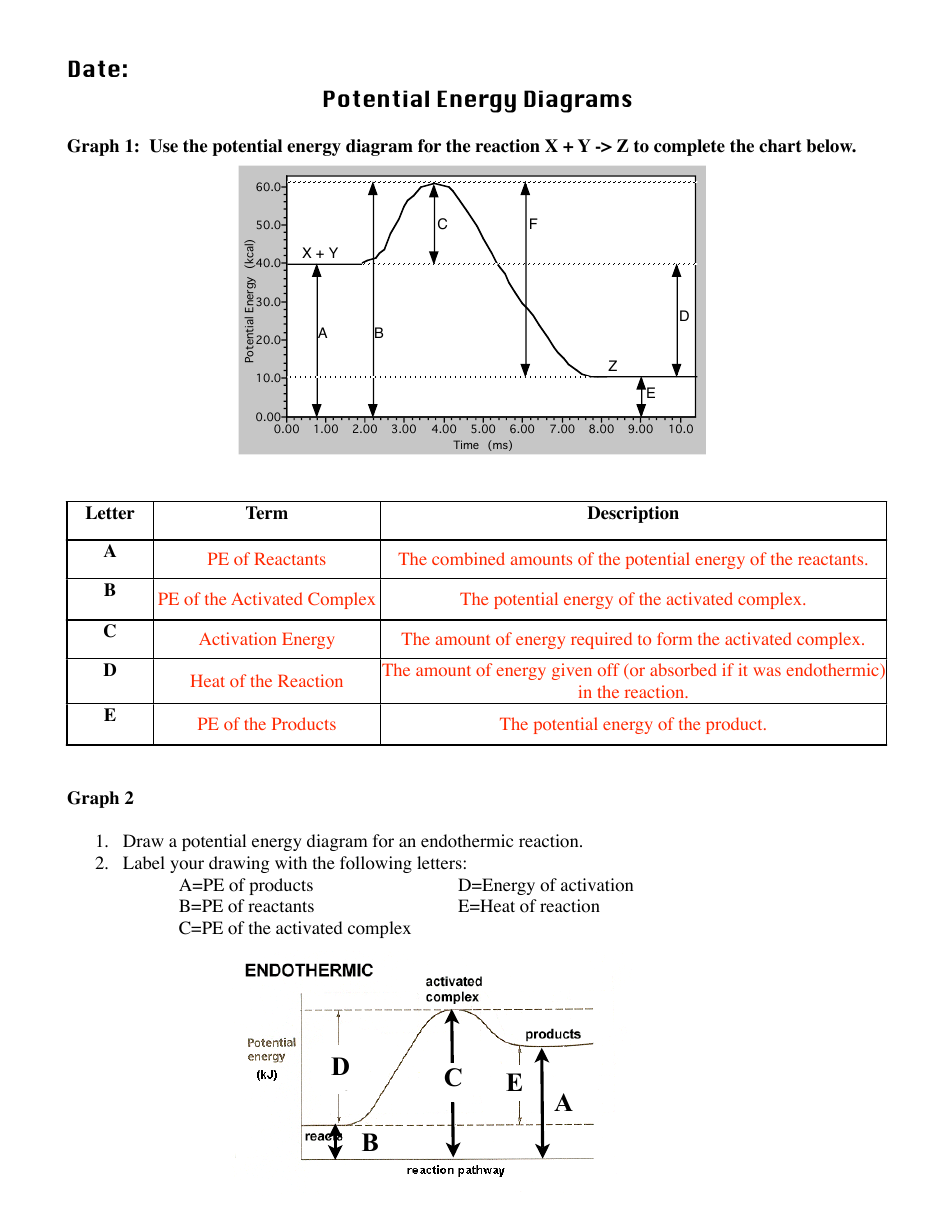
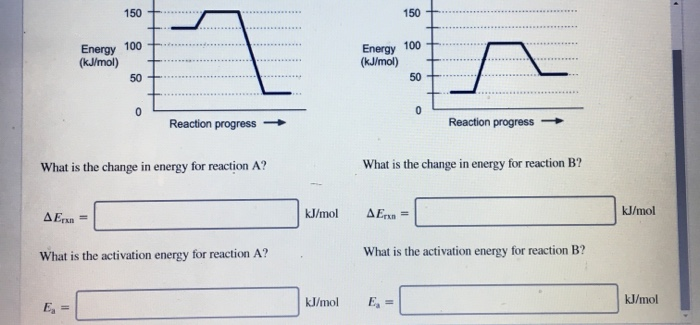
Use this energy diagram to answer these questions. 1. The enthalpy of the reactants of the reaction is about kilojoules. 2. The enthalpy of the products of the reaction is about kilojoules. 3. The activation energy of the reaction is about kilojoules. 4. The heat of reaction (ΔH) of the reaction is about kilojoules.
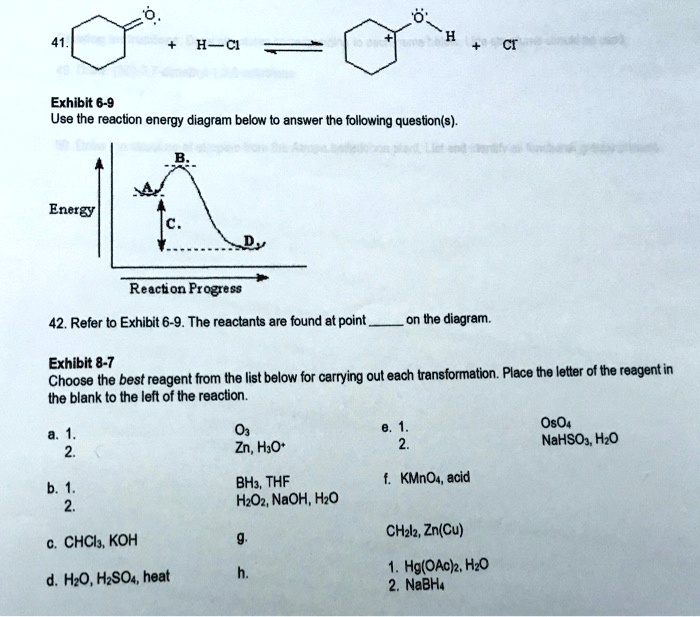
Instructions: Use the reaction energy diagram below to answer the following question(s).-The following group is a substituent on a molecule. What is an accepted IUPAC name for this group? A) propenyl B) allyl C) vinyl D) propylene E) either a or b
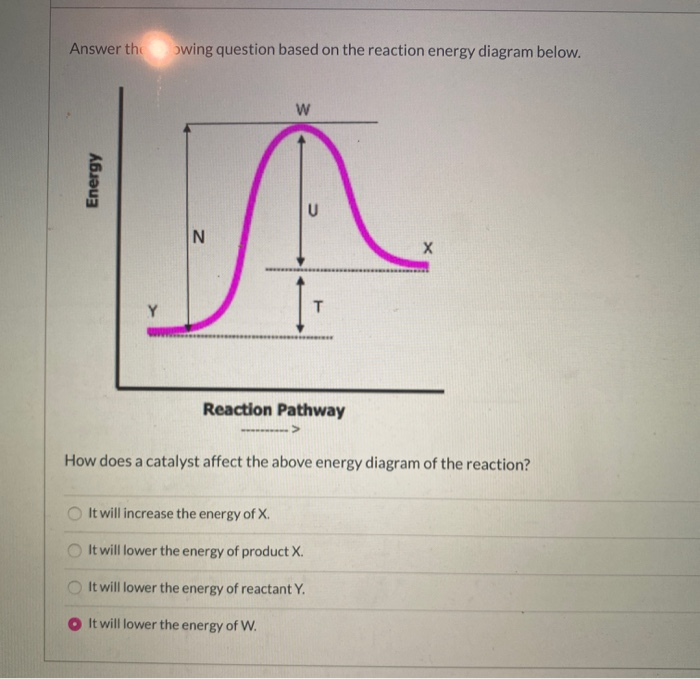
Increasing temperature, increases reaction rate. When E, is large, the rate constant k is also large. Energy. Considering below energy diagram, which of the following statement is true? Reaction coordinate Fast reactions have equilibrium constants > 1. A fast reaction has a large negative AG° value. Increasing temperature, increases reaction rate.
Use the potential energy diagram below to answer the following question The potential energy of the reactants is ____kJ, while the potential energy of the products is _____. ( straight, wayyyyyyyyyyy uphill, then tiny downhill, then straight )
I can’t think of how to answer this question. Any help would be great!!
Reaction Rates and Potential Energy Diagrams 1. Chemical reactions occur when reactants collide. For what reasons may a collision fail to produce a chemical reaction? Not enough energy; improper angle. 2. If every collision between reactants leads to a reaction, what determines the rate at which the reaction occurs?
Match. Gravity. Refer to the free energy diagrams below to answer the following questions. You may assume that the y-axis is the same and directly comparable for all four reactions. Click card to see definition 👆. Tap card to see definition 👆.
The diagram represents a spontaneous reaction. Use the diagram to answer the questions below. a. Is the reaction endothermic or exothermic? b. What is the activation energy of the reaction?
Name: Directions: Use the graph to answer the questions below 1. Does this graph represent an exothermic reaction or an endothermic reaction? Explain how you know Exothermic; From the potential energy diagram, it is observed that the reactants A and B have an energy value of 40 kJ. The products C and D have a lowered energy value of 20 kJ. The ...
In a world where the use of magic typically means drawing power from your own body heat, a particularly crafty mage discovers a way to use a controlled nuclear fission reaction as a source of energy. What follows is an epic, action packed fight for survival. In the world of Fables, when a Fable is born a Fable becomes the very embodiment of the magic that created it. The Fable game book is available here on Amazon US, Amazon UK, Barnes and Noble US, and Barnes and Noble UK. Here's the cover: ...
Use the reaction energy diagram above to answer the following questions. Calculate the activation energy, ΔG for the step C to B _____ kcal/mol Calculate the overall energy change, ΔG°, for the process B to A. _____kcal/mol. Which step is faster, (a) B to A or (b) B to C?
Use the reaction energy diagram above to answer the following questions. Calculate the activation energy, ΔG, for the step A to B. kcal/mol. Calculate the overall energy change, ΔG°, for the process B to C. kcal/mol. Which step is faster, (a) A to B or (b) C to B. fullscreen Expand.
This might only work on mobile and on android.
Problem Details. Use the energy diagram for the reaction A → D to answer the questions. Q. Which of the following has ΔG°f = 0 at 25°C?a. O3 (g)b. O (g)c. H2O (g)d. H2O (l)e. Na (s) Q. Predict the sign of ΔG for an endothermic reaction with adecrease in entropy. a)cannot predict b)positive c)negative d)no change e)Aquarius.
Question 15. SURVEY. 30 seconds. Q. How much energy is released when two moles of methane (CH 4) reacts at 298 K and 101.3 kPa according to the following equation: CH 4 (g) + 2O 2 (g) → CO2(g) + 2H2O (l) answer choices. 890.4 J. 890.4 kJ.
Potential Energy Diagrams Worksheet CK-12 Foundation Chemistry Name Use the following Potential Energy Diagram to answer questions 1 - 12. 150 X2Y2 100 Potential Energy (kJ) 50 0 Progress of Reaction 1. Is the overall reaction as shown exothermic or endothermic? 2. What is the activation energy for the forward reaction? 3. What is the ...
Hello there! I am on a bulk. I've never really found a proper answer to this, so I've come here now. When I do cardio, I make sure to eat the amount of calories that I've burned. Should I do the same when weightlifting, or is that the reason you eat in a surplus of +500 calories? ​ Cheers!

















0 Response to "38 use the energy diagram for the reaction to answer the questions."
Post a Comment