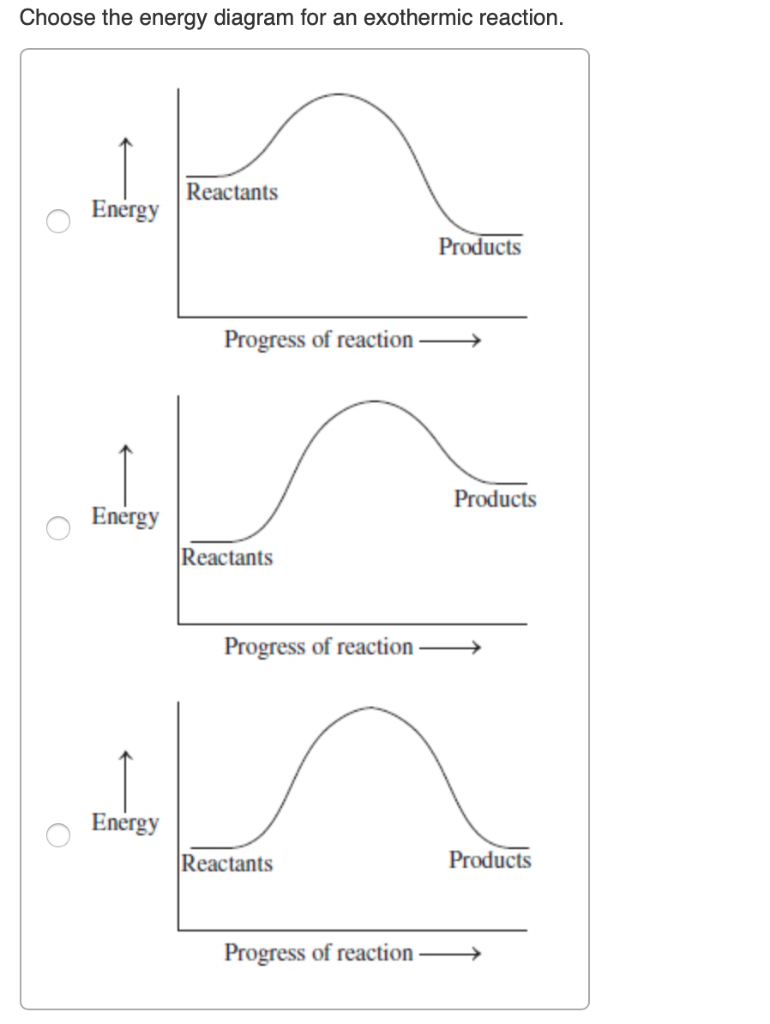
38 energy diagram endothermic and exothermic reaction
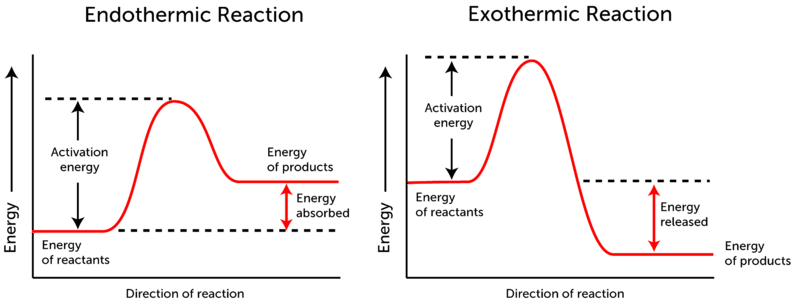
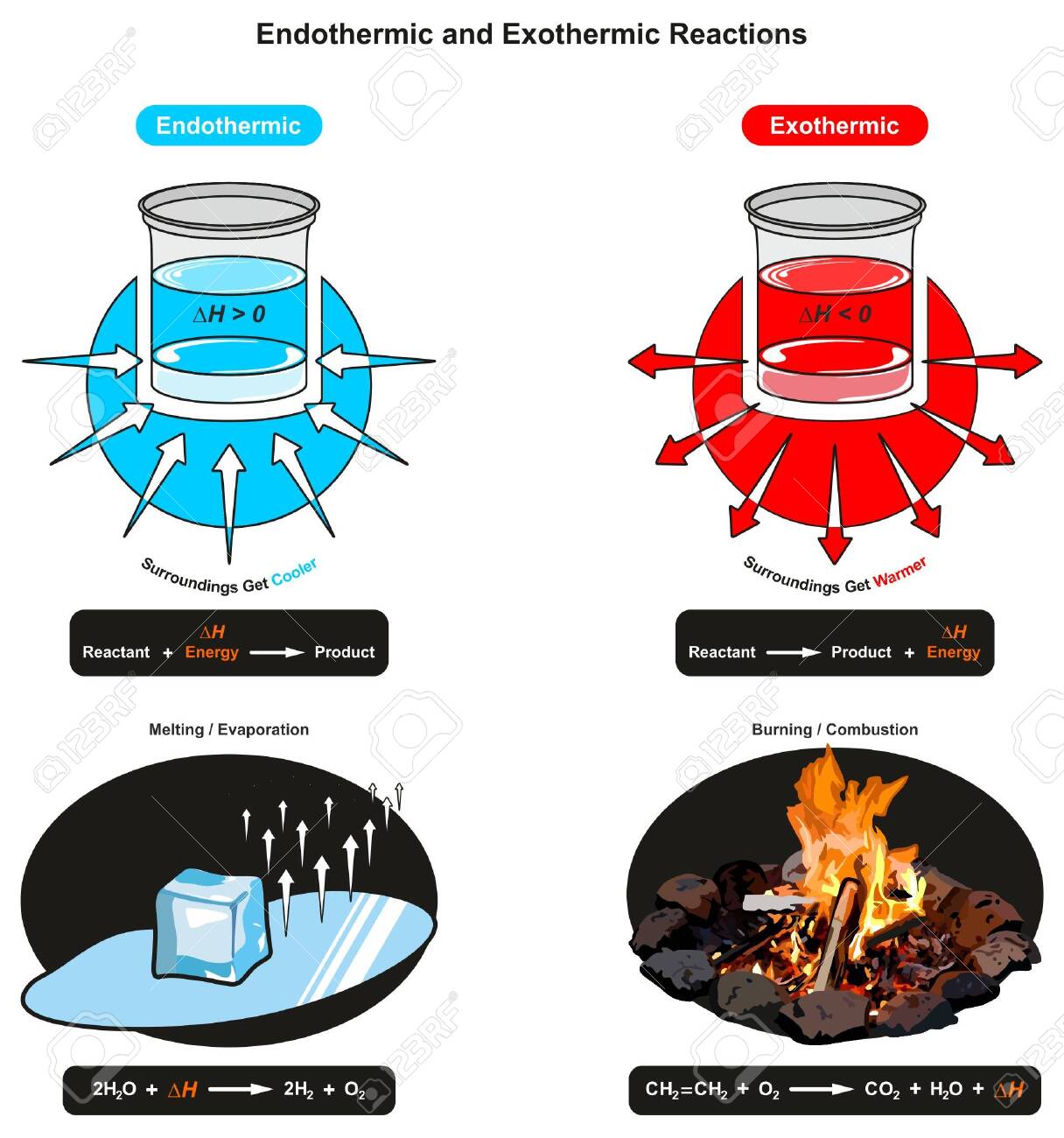
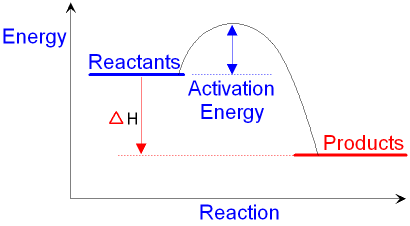
Endothermic Diagram. Energy absorbed in reaction. Activation . Energy. Energy used in bond. breaking. Endothermic – more energy is taken in to break the bonds in the reactants than released by the bonds being formed in the products. Therefore, energy is absorbed. Energy released in bond making In the case of an endothermic reaction, the reactants are at a lower energy level compared to the products—as shown in the energy diagram below. In the case of an exothermic reaction, the reactants are at a higher energy level as compared to the products, as shown below in the energy diagram.
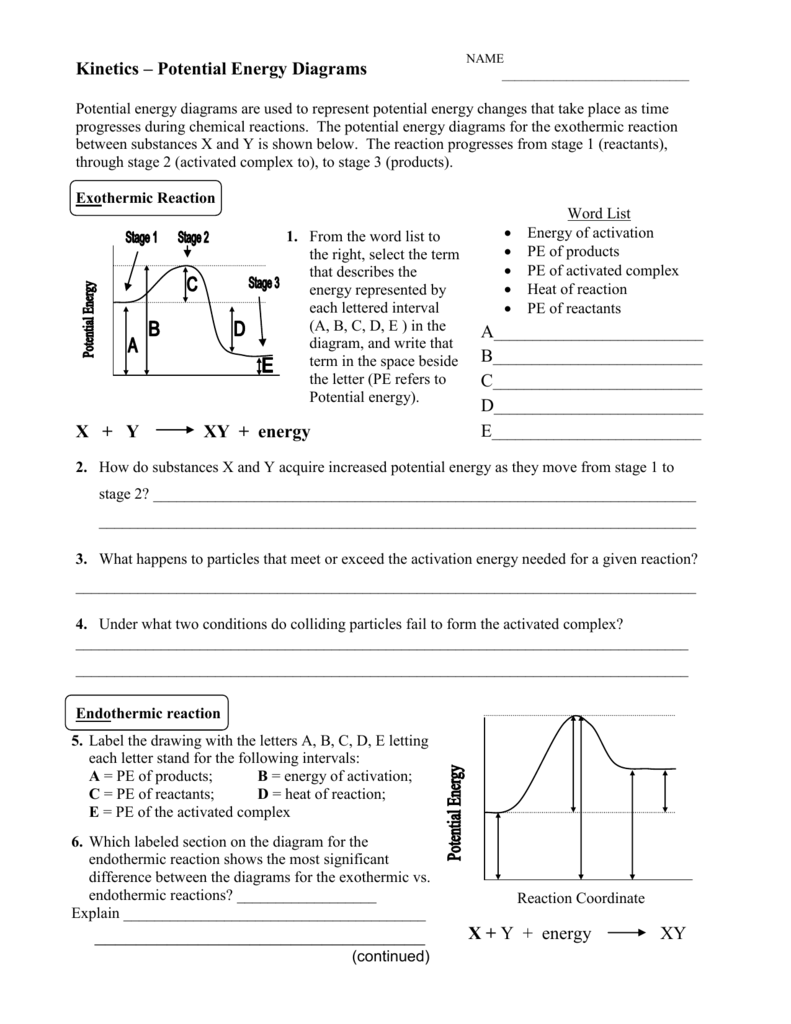
Energy Diagrams. Endothermic and exothermic reactions can be visually represented by energy-level diagrams like the ones in Figure \(\PageIndex{2}\). In endothermic reactions, the reactants have higher bond energy (stronger bonds) than the products. Strong bonds have lower potential energy than weak bonds.

Energy diagram endothermic and exothermic reaction
Energy level diagrams for exothermic reactions In an exothermic reaction, reactants have more energy than the products . The difference between these two energy levels is the energy released to the surroundings, shown as a vertical drop from a higher to a lower level. Because the reactants have more energy than the products they are less stable. An energy level diagram. shows whether a reaction is exothermic. or endothermic. It shows the energy in the reactants and products , and the difference in energy between them. Exothermic reaction A reaction that takes in heat energy so the temperature goes down on a energy profile diagram is it a exothermic or endothermic reaction if the activation energy is small. In the case of an endothermic reaction the reactants are at a lower energy level compared to the productsas shown in the energy diagram below.
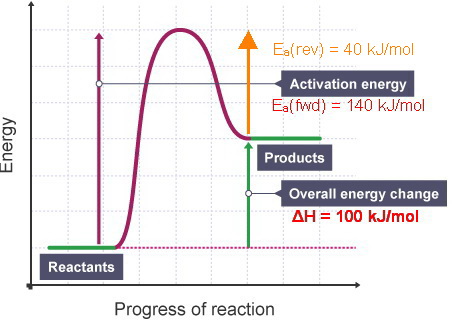
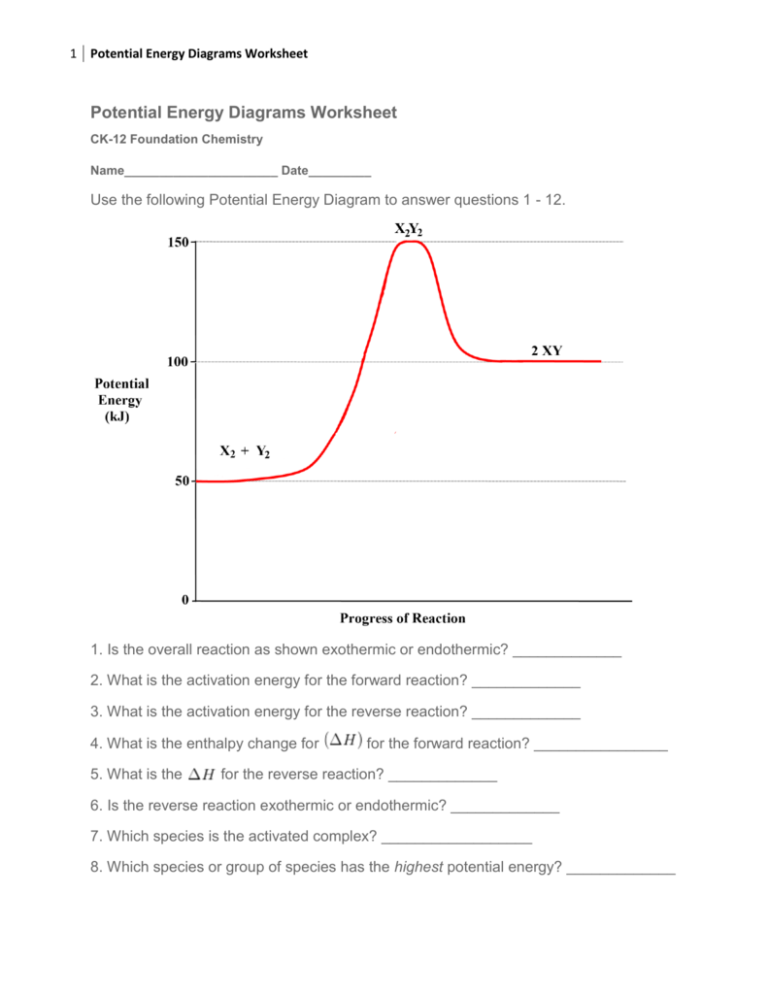
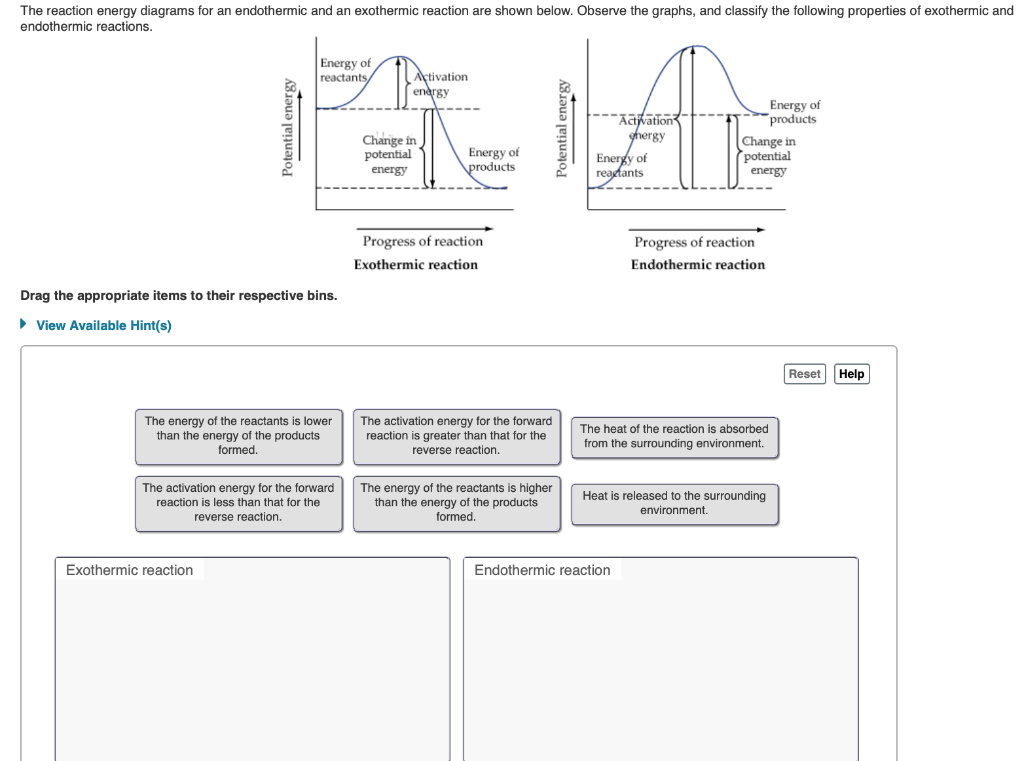
Energy diagram endothermic and exothermic reaction. Transcribed image text: The reaction energy diagrams for an endothermic and an exothermic reaction are shown below. Observe the graphs, and classify the following properties of exothermic and endothermic reactions. Energy of reactants Activation energy Potential energy Potential energy Change in Energy of products Change in potential energy Activation ghergy Energy of reactants potential ... A general Reaction Coordinate Diagram relating the energy of a system to leading to an exothermic reaction (∆H 0). Endothermic Reaction. One of your salts generated an endothermic reaction with water, while the other salt generated an . Energy diagrams for endothermic and exothermic reactions. In endothermic reactions, there is less energy in the reactants than in the products. Activation Energy The activation energy in an exothermic reaction is energy at the top of the curve minus the energy of the reactants. Aug 22, 2020 · Enthalpy, or heat energy, is represented by ΔH (Δ is the delta sign, which means change). If there is a negative change in energy, or -ΔH, an exothermic reaction is taking place and energy is released🔥 from the system to the surroundings. If there is a positive change in energy, or +ΔH, an endothermic reaction is taking place and energy is absorbed into the system from the surroundings.
The heat of reaction is also called enthalpy change and symbolized by∆H, the energy absorbed or released in a reaction. In an endothermic reaction, energy is absorbed. Sign of ∆H is positive. If written separately, on the reactant side if mentioned in the reaction. On the energy diagram products are on the higher energy level than the ... Energy Diagrams. Endothermic and exothermic reactions can be visually represented by energy-level diagrams like the ones in Figure \(\PageIndex{2}\).In endothermic reactions, the energy of the reactants is lower than that of the products.This type of reaction is represented by an "uphill" energy-level diagram shown in Figure \(\PageIndex{2A}\). A reaction that takes in heat energy so the temperature goes down on a energy profile diagram is it a exothermic or endothermic reaction if the activation energy is small. In the case of an endothermic reaction the reactants are at a lower energy level compared to the productsas shown in the energy diagram below. An energy level diagram. shows whether a reaction is exothermic. or endothermic. It shows the energy in the reactants and products , and the difference in energy between them. Exothermic reaction
Energy level diagrams for exothermic reactions In an exothermic reaction, reactants have more energy than the products . The difference between these two energy levels is the energy released to the surroundings, shown as a vertical drop from a higher to a lower level. Because the reactants have more energy than the products they are less stable.























0 Response to "38 energy diagram endothermic and exothermic reaction"
Post a Comment