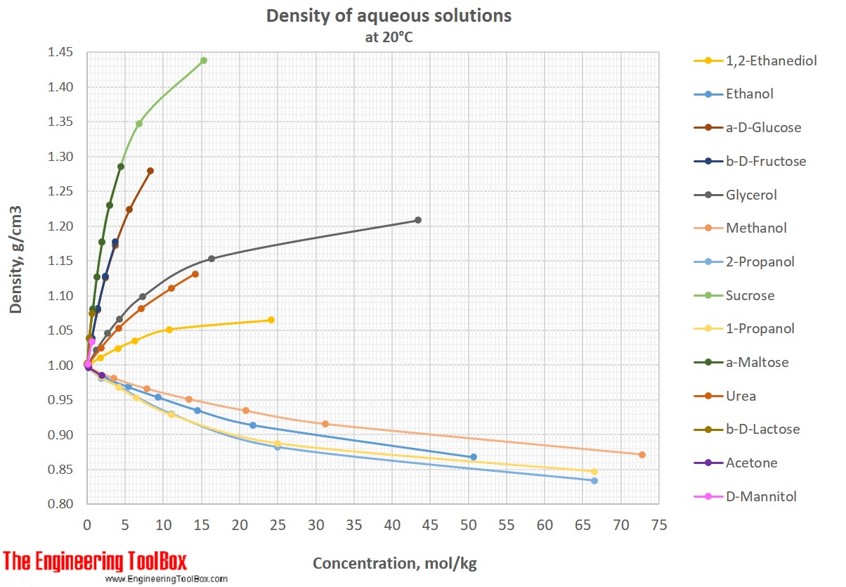
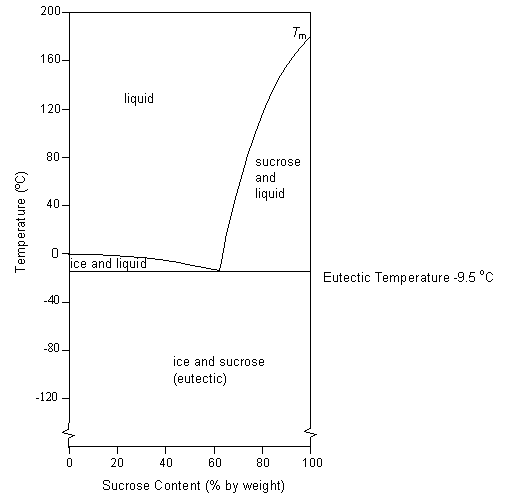
36 consider the sugar-water phase diagram
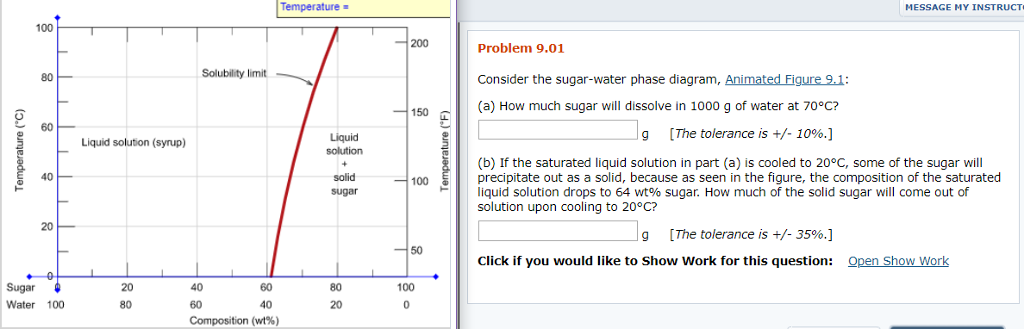
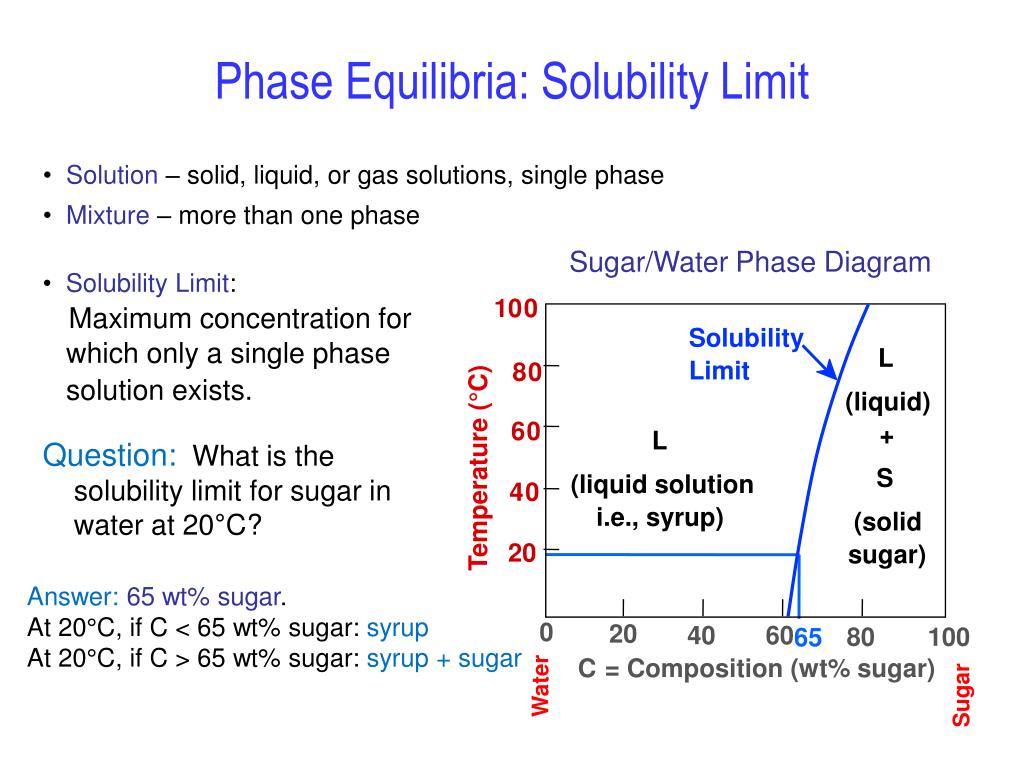
Transcribed image text: Consider the sugar-water phase diagram, Animated Figure 10.1: (a) How much sugar will dissolve in 1000 g of water at 70°C? i 5050 g (b) If the saturated liquid solution in part (a) is cooled to 20°C, some of the sugar will precipitate out as a solid, because as seen in the figure, the composition of the saturated liquid solution drops to 64 wt% sugar. Consider the sugar-water phase diagram of Figure 9.1. (a) How much sugar will dissolve in 1000 g of water at 80°C (176°F)? (b) If the saturated liquid solution in part (a) is cooled to 20°C (68°F), some of the sugar will precipitate out as a solid.
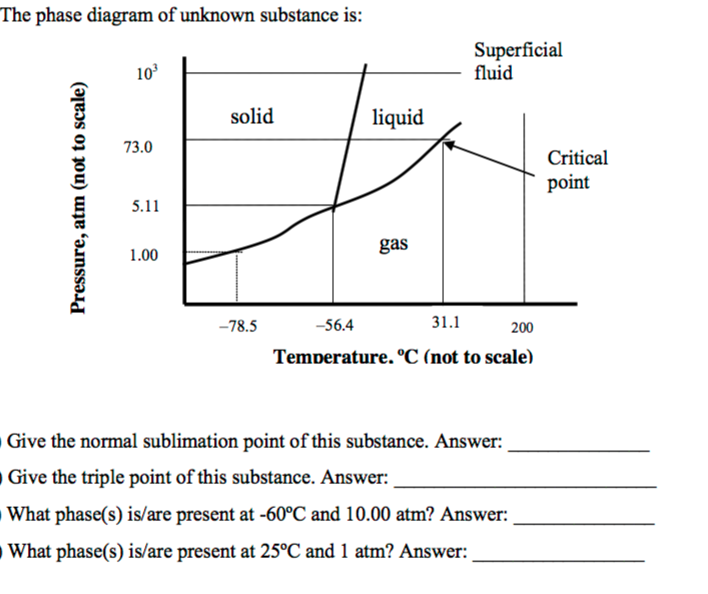
The standard state of a chemical substance is its phase (solid, liquid, gas) at 25.0 °C and one atmosphere pressure. This temperature/pressure combo is often called "room conditions." Determine the specific heat capacity of an alloy that requires 59.3 kJ to raise the …
Consider the sugar-water phase diagram
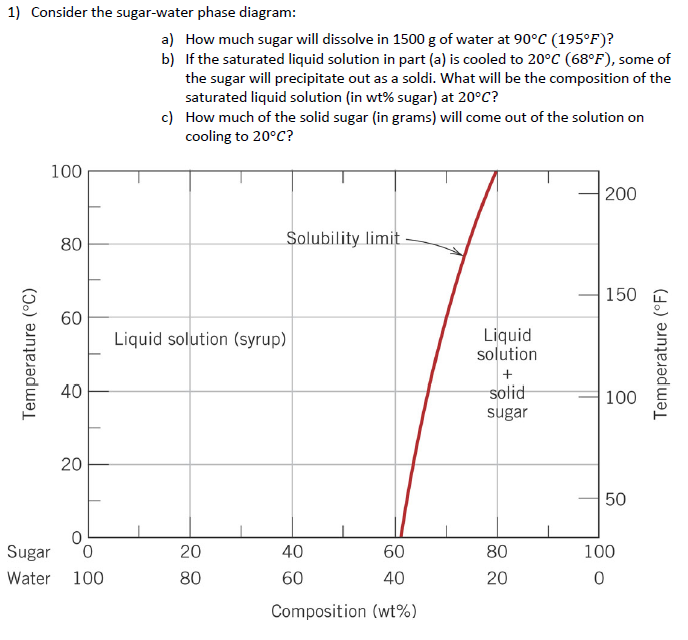
Science; Chemistry; Chemistry questions and answers; Consider the sugar-water phase diagram, Animated Figure 10.1 (a) How much sugar will dissolve in 1000 g of water at 70°C? e (b) If the saturated liquid solution in part (a) is cooled to 20°C, some of the sugar will precipitate out as a solid, because as seen in the figure, the composition of the saturated liquid solution drops to 64 wt% sugar. 9.1 Consider the sugar-water phase diagram of Figure 9.1. (a) How much sugar will dissolve in 1500 g of water at 90°C (194°F)? (b) If the saturated liquid solution in part (a) is cooled to 20°C (68°F), some of the sugar will precipitate out as a solid. This phase occurs when the community organization has already been established and the community members are already participating in the community-wide. a. Pre-entry phase c. Organizational building phase b. Entry phase d. Sustenance and strengthening phase 43. The following are guidelines that should be followed in the entry phase, except: a.
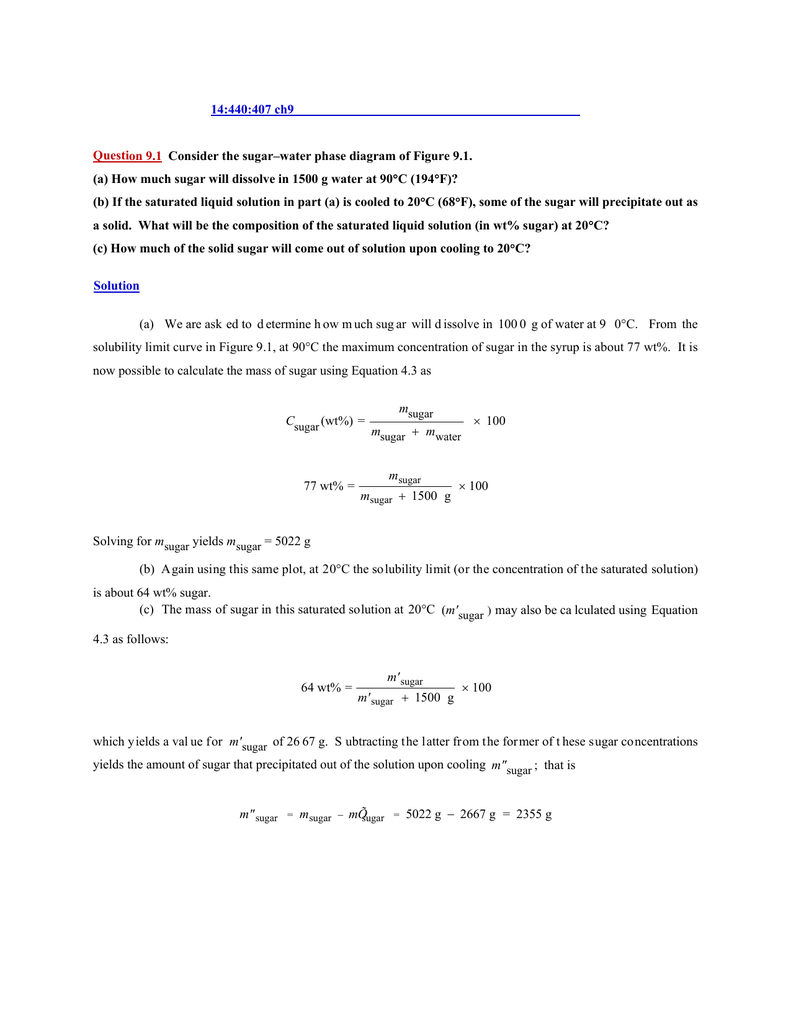
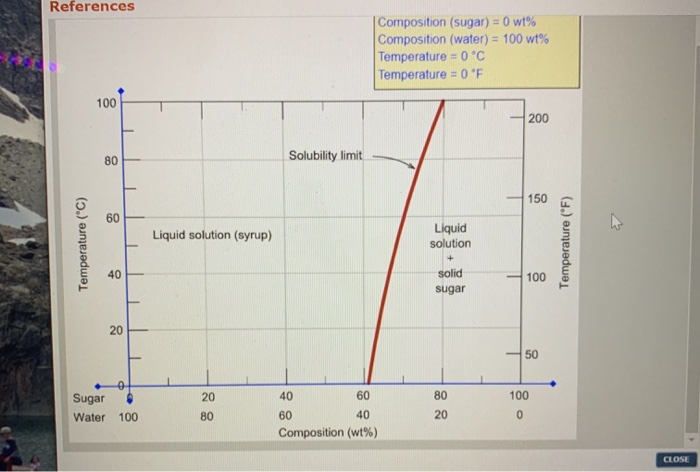
Consider the sugar-water phase diagram. PHASE DIAGRAMS PROBLEM SOLUTIONS Solubility Limit 9.1 Consider the sugar–water phase diagram of Figure 9.1. (a) How much sugar will dissolve in 1500 g water at 90 °C (194 °F)? (b) If the saturated liquid solution in part (a) is cooled to 20 °C (68 °F), some of the sugar will precipitate out as a solid. Consider a sugar-water phase diagram: (a) How much sugar will dissolve in 1000 g of water at 90{eq}^o {/eq} C? (b) If the saturated liquid solution in part (a) is cooled to 20{eq}^o {/eq} C, some ... Phase Equilibria: Solubility Limit 65 Sucrose/Water Phase Diagram Pure Sugar emperature (°C) 0 20 40 60 80 100 C o =Composition (wt% sugar) L (liquid solution i.e., syrup) Solubility Limit L (liquid) + S (solid 20 sugar) 4 0 6 0 8 0 10 0 Pure ater Question: What is the solubility limit at 20C? Answer: 65wt% sugar. If Co < 65wt% sugar: syrup If ... Yes. Our services are very confidential. All our customer data is encrypted. We consider our client’s security and privacy very serious. We do not disclose client’s information to third parties. Our records are carefully stored and protected thus cannot be accessed by unauthorized persons. Our payment system is also very secure.
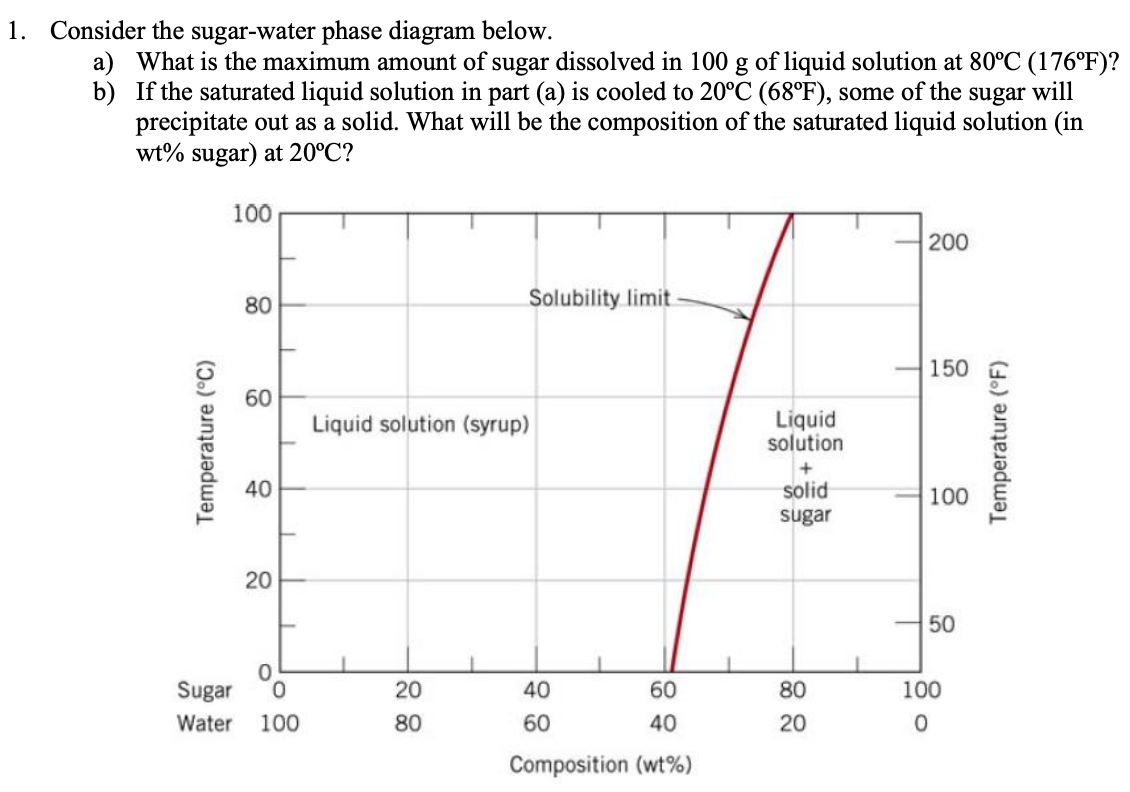
Academia.edu is a platform for academics to share research papers. Yes. Our services are very confidential. All our customer data is encrypted. We consider our client’s security and privacy very serious. We do not disclose client’s information to third parties. Our records are carefully stored and protected thus cannot be accessed by unauthorized persons. Our payment system is also very secure. Consider the sugar-water phase diagram below. a) What is the maximum amount of sugar dissolved in 100 g of liquid solution at 80°C (176°F)? b) If the saturated liquid solution in part (a) is cooled to 20°C (68°F), some of the sugar will precipitate out as a solid. Yes. Our services are very confidential. All our customer data is encrypted. We consider our client’s security and privacy very serious. We do not disclose client’s information to third parties. Our records are carefully stored and protected thus cannot be accessed by unauthorized persons. Our payment system is also very secure.
Nov 20, 2021 · Lesson 1 what is a mixture answer key 9.1 Consider the sugar–water phase diagram of Figure 9.1. (a) How much sugar will dissolve in 1500 g water at 90°C (194°F)? (b) If the saturated liquid solution in part (a) is cooled to 20°C (68°F), some of the sugar will precipitate out as a solid. What will be the composition of the saturated liquid solution (in … Answer: 65 wt% sugar . If Co < 65 wt% sugar: syrup If Co > 65 wt% sugar: syrup + sugar. 65 Sucrose/Water Phase Diagram Pure Sugar Temperature (°C) 0 20 40 60 80 100 Co =Composition (wt% sugar) L (liquid solution i.e., syrup) Solubility Limit L (liquid) + S (solid 20 sugar) 40 60 80 100 Pure Water Adapted from Fig. 9.1, Callister 7e. Q.1. Consider the sugar-water phase diagram of Figure 9.1. (a) How much sugar will dissolve in 1500 g water at 90°C (363 K)? (b) If the saturated liquid solution in part (a) is cooled to 20°C (293K), some of the sugar will precipitate out as a solid. What will be the composition of the saturated liquid solution (in wt% sugar) at 20°C? Q.2.
This phase occurs when the community organization has already been established and the community members are already participating in the community-wide. a. Pre-entry phase c. Organizational building phase b. Entry phase d. Sustenance and strengthening phase 43. The following are guidelines that should be followed in the entry phase, except: a.
9.1 Consider the sugar-water phase diagram of Figure 9.1. (a) How much sugar will dissolve in 1500 g of water at 90°C (194°F)? (b) If the saturated liquid solution in part (a) is cooled to 20°C (68°F), some of the sugar will precipitate out as a solid.
Science; Chemistry; Chemistry questions and answers; Consider the sugar-water phase diagram, Animated Figure 10.1 (a) How much sugar will dissolve in 1000 g of water at 70°C? e (b) If the saturated liquid solution in part (a) is cooled to 20°C, some of the sugar will precipitate out as a solid, because as seen in the figure, the composition of the saturated liquid solution drops to 64 wt% sugar.















0 Response to "36 consider the sugar-water phase diagram"
Post a Comment