37 mo diagram for nh3
Molecular Orbital Theory - Walsh diagram The Walsh diagram shows what happens to the molecular orbitals for a set of molecules which are related in structure. In this case, the difference is the H-X-H bond angle which decreases from 180 o to 90 o Molecular Orbital Theory - Walsh diagram Water 104.5 ° X H H H O H MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine
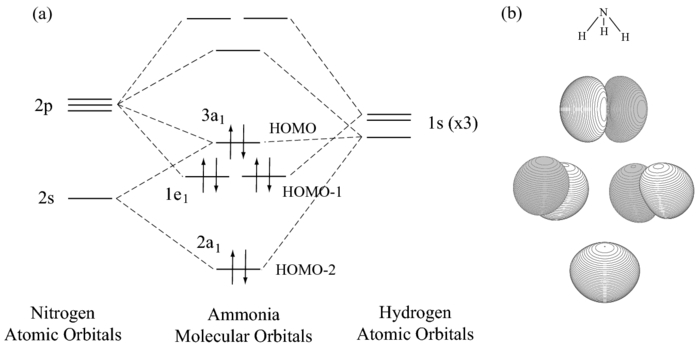
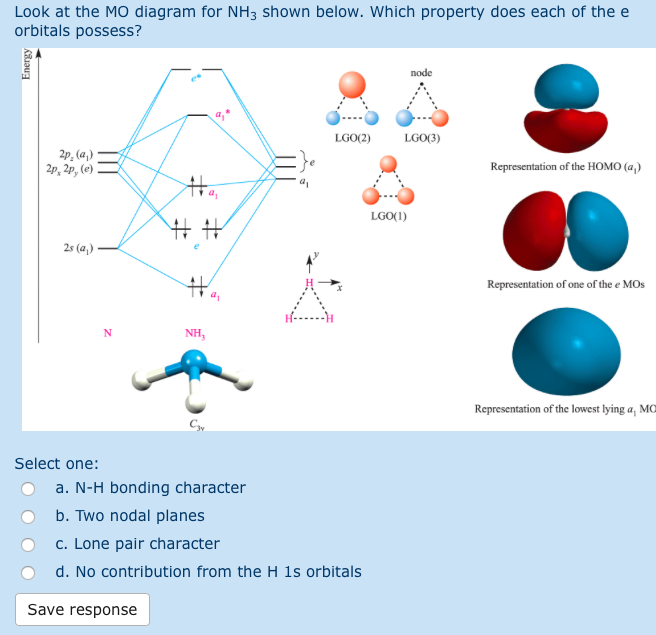
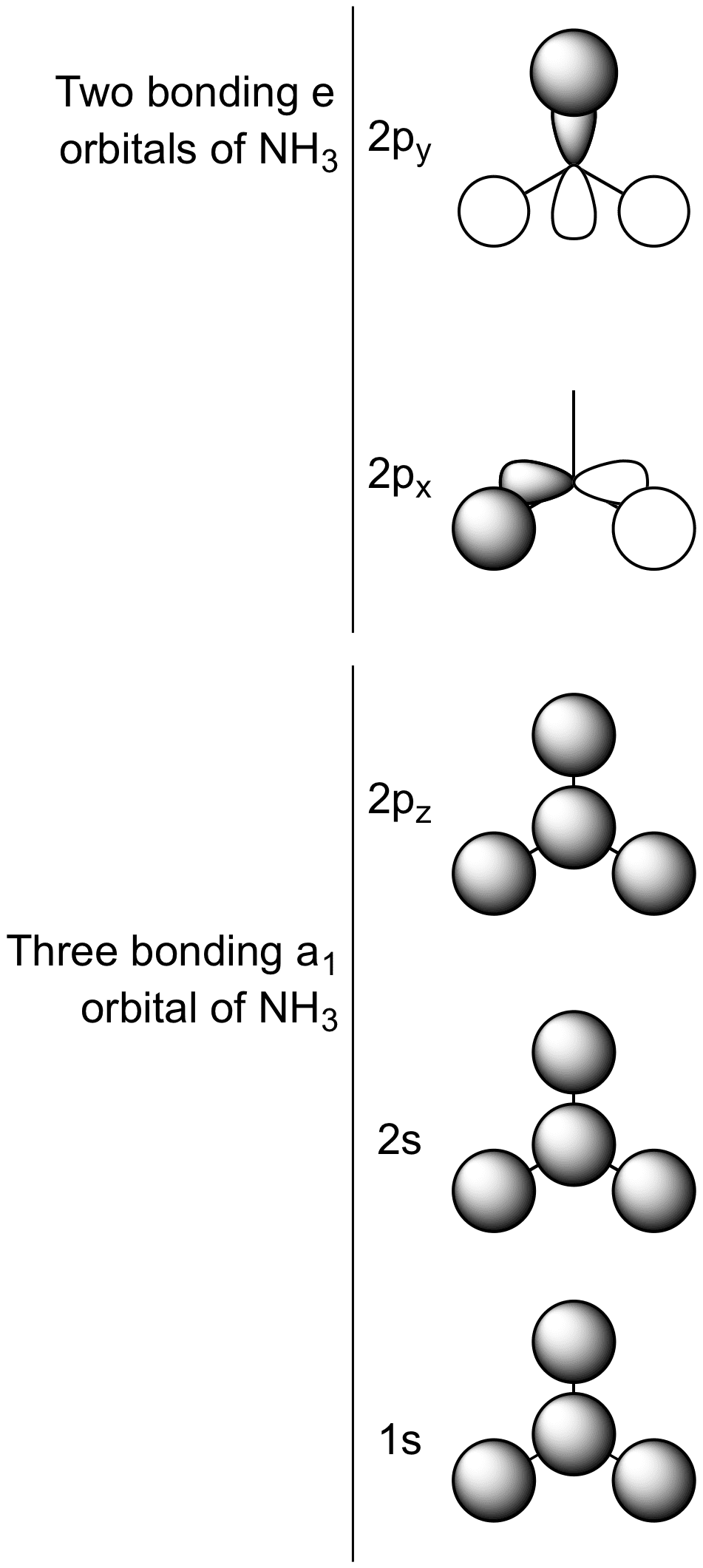
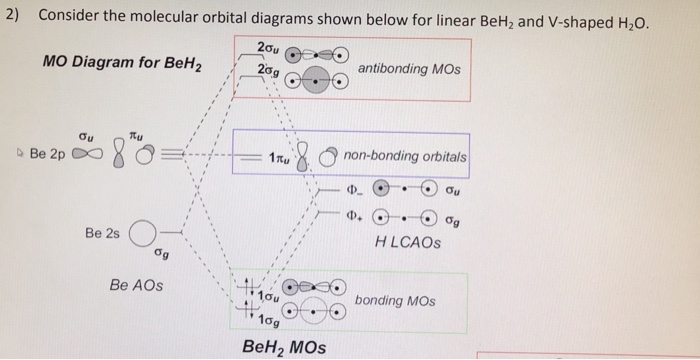
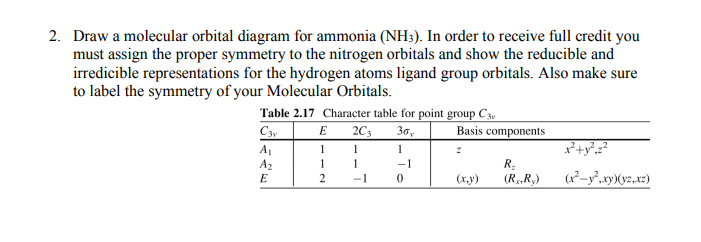
The molecular orbital diagram of NH3 is presented in Figure 5 and will be elaborated in regards to its interactions. The s orbitals for the 3 hydrogens are used to set up the sigma and anti bonding combinations of N sp 3 orbitals and the H 1s orbitals. Derive the molecular orbital diagrams for linear and bent H 2 O.
Mo diagram for nh3
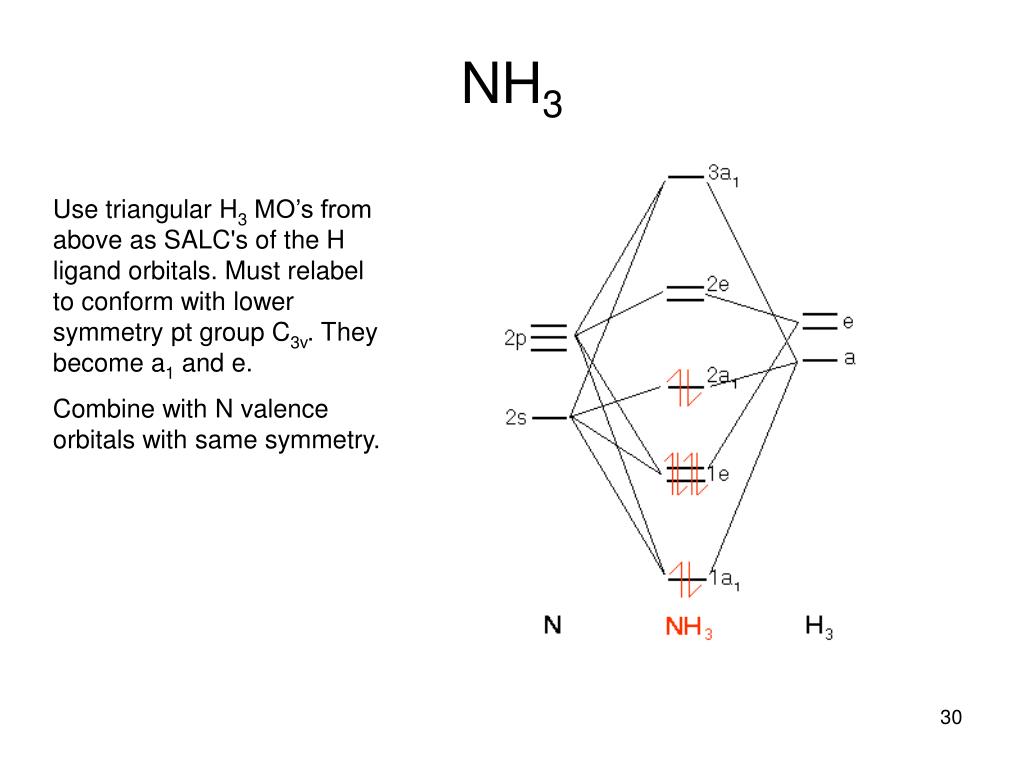
use qualitative MO theory to predict if BeH2 will be bent or linear. ... (do AFTER the problems class) draw a MO diagram for planar D3h NH3. In contrast to crystal field theory, molecular orbital included the covalent nature of the metal-ligand bond interaction. No Metal- Ligand -bonding ( bonding only) Let's take [Co(NH3)6]3+ as an example. Using the LGO method, one can construct a qualitative MO diagram for bonding in a [ML6]n+ complex. A BRIEF INTRODUCTION TO MOLECULAR ORBITAL THEORY OF SIMPLE POLYATOMIC ... the polyatomic molecules H2O, NH3, BH3 and SiH4 using group theory is reported.
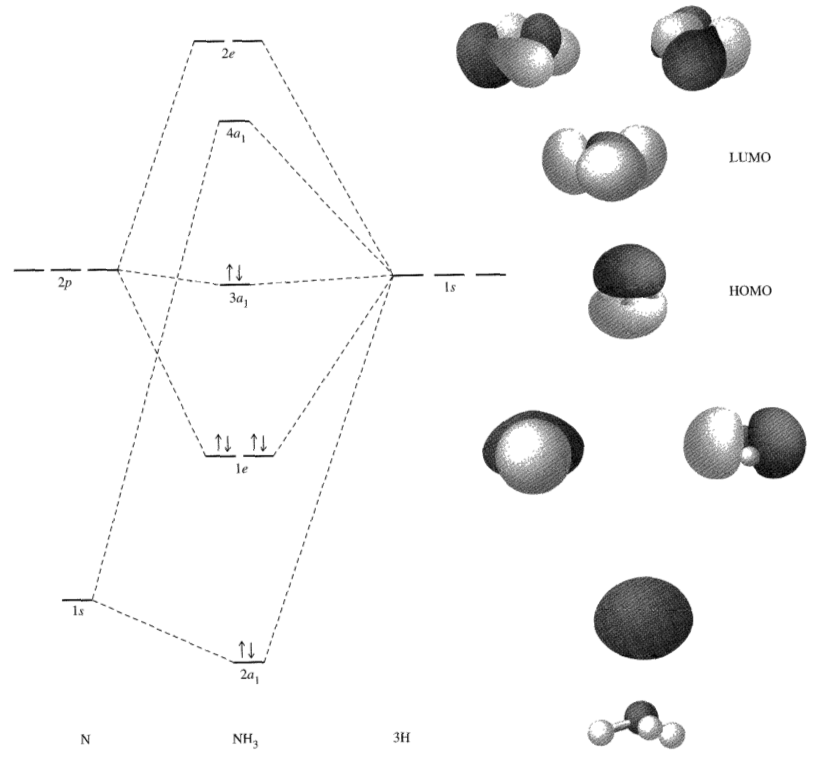
Mo diagram for nh3. The molecular orbital diagram of NH3 is presented in Figure 5 and will be elaborated in regards to its interactions. Drawing and calculating molecular orbitals of ammonia. MO diagram in Figure - a1 symmetry orbitals • Bondings, nonbonding, and antibonding MO - e. In NH3, the HOMO (Highly Occupied Molecular Orbital) is a mostly nitrogen based orbital that corresponds to the Lone pair of electrons. This is why ammonia acts as a Lewis base at the N atom. The LUMO (Lowest Unoccupied Molecular Orbital) is the 2e level that has more H character this shows why NH3 can also act as a Lewis acid through the H atoms. Construct a molecular orbital diagram for the valence electrons of NH3 by listing the valence atomic orbitals of nitrogen on the left hand side and ligand group orbitals on the right hand side. Combine those orbitals that have similar symmetry and arrange the molecular orbitals in the center of the diagram. Nh3 Molecular orbital Diagram. molecular orbitals of ammonia molecular orbital in nh3 the homo highly occupied molecular orbital is a mostly nitrogen based orbital that corresponds to the lone pair of electrons this is why ammonia acts as a lewis base at the n atom lecture 10 part b mo diagram of nh3 che 422 inorganic chemistry molecular orbital energy level diagram of nh3 and frontier ...
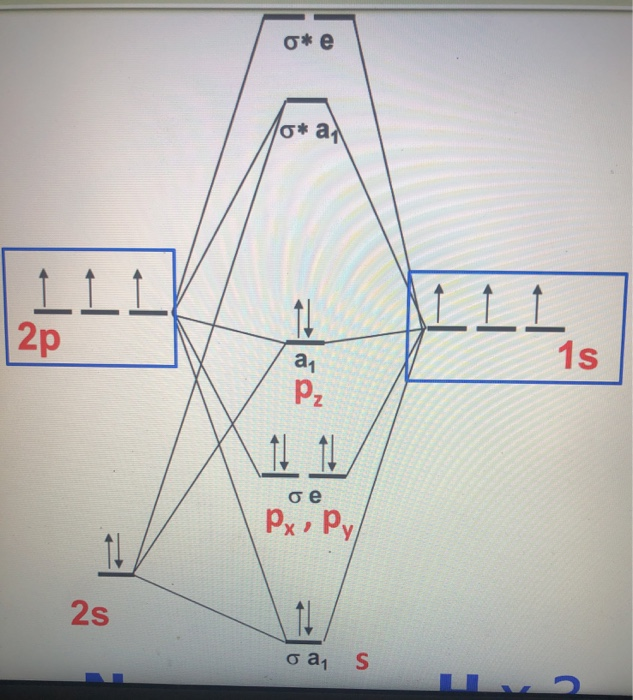
The MO energy diagram for NH3 is shown in Figure 3. The energy. As can be seen from the energy diagram - four of the molecular orbitals occur as Ammonia has two pairs of degenerate orbitals, one bonding and one.MO Diagram for Triangular H 3 A fragment approach to deriving molecular orbitals Inorganic Chemistry. -35-(b) The P.E.S. results are consistent with the MO scheme. Only the Bn(x) MO is strictly nonbonding, while the F(z) MO is weakly bonding, as indicated by the vibrational fine structureon its P.E.S. band. (c) Rather than two lone pairs in approximately sp3 hybrids, the MO scheme suggests a single region of electron density protruding from the back side of the molecule. NF3 Lewis Structure, Molecular Geometry, Hybridization, Polarity, and MO Diagram. Nitrogen trifluoride or NF3 is a nitrogen halide compound that is slightly water-soluble. Its noticeable characteristics include being colorless and carrying a musty or moldy odor. NF3 has a molar mass of around 71.002 g/mol and a density of 3.003 kg/m3. The energy level diagrams are shown in Figures 1, 2, 3a, b. It should be pointed out that the dotted lines connecting the a.o.'s to the m.o.'s represent a one electron partici- pation (or population) of greater than 10%. ... Energy levels for [Ni(NH3)6]:+ Fu =1.3. Shupack I Molecular Orbital Theory for Metal-Ammine Complexes \ a, e t,. 442 ...
CHE 422 Inorganic Chemistry. Molecular orbital energy level diagram of NH3 and Frontier Orbitals (highest occupied molecular orbital HOMO and lowest unoccup... Answer: I think you can safely assume to start off with the molecular orbital diagram of the Nitrite anion (NO₂¯) and then remove an electron from it: What will be the molecular orbital diagram for nitrite ion? The outcome, i.e. the molecular orbital diagram for Nitrogen dioxide NO₂, should loo... Nh3 Molecular Orbital Diagram. As can be seen from the energy diagram - four of the molecular orbitals occur as Ammonia has two pairs of degenerate orbitals, one bonding and one. MO diagram of homonuclear diatomic molecules. • Filling the . 4) MO theory and molecular geometry (Walsh diagrams) . 2) Molecular Orbitals of NH3 (C3v). It should be possible, I suppose, to argue for why this is so by using qualitative molecular orbital theory, and from looking at the construction of the molecular orbital diagrams, but I am not sure how to construct accurate MO diagrams for $\ce{PH3}$ and $\ce{NH3}$. In the answer to the possible duplicate question, it seems to be taken for ...
Using the Molecular Orbital Model to Explain Why Some Molecules Do Not Exist. This molecular orbital model can be used to explain why He 2 molecules don't exist. Combining a pair of helium atoms with 1s 2 electron configurations would produce a molecule with a pair of electrons in both the bonding and the * antibonding molecular orbitals. The total energy of an He 2 molecule would be ...
NH3 Lewis Structure, Molecular Geometry, Hybridization, Bond Angle & Shape. Ammonia is a colorless compound, used in making fertilizers. It is a stable hydride formed of one nitrogen and three hydrogen atoms. The molecule has a pungent smell. It can form an NH4+ ion by accepting a proton.
Solved Using A Simple Mo Approach Explain What Happens To The Energies Of The Metal D Orbitals On The Formation Of A Bonded Complex Such As Ni Course Hero
It is recommended to name the SVG file "Ammonia MO diagram.svg" – then the template Vector ... English: MO energy level diagram for the ammonia molecule.
27 Sept 2021 ... MO diagram for NH · Viewed end-on, a p-orbital or an spx hybrid orbital looks just like an s-orbital. · We now construct the sp3 hybrid orbitals ...
Molecular orbital diagram of NH3. Ammonia or NH3 has 8 valence electrons, consisting of a lone pair on its nitrogen and 3 N-H sigma ...
Follow me on instagram-https://www.instagram.com/trickychemistrysuman/?hl=enFollow me on facebook page-https://lm.facebook.com/l.php?u=https%3A%2F%2Ffb.me%2F...
MO diagram. Overall symmetry D ∞h. The SALC's for the 2 Cl atoms will be the Cl + Cl and Cl - Cl combinations. c. If the Cl pi donor orbitals are considered, their SALC's will be and , plus the orthogonal pair. The first of these will mix with the metal p orbitals to make bonding and antibonding combinations, but neither of these will ...
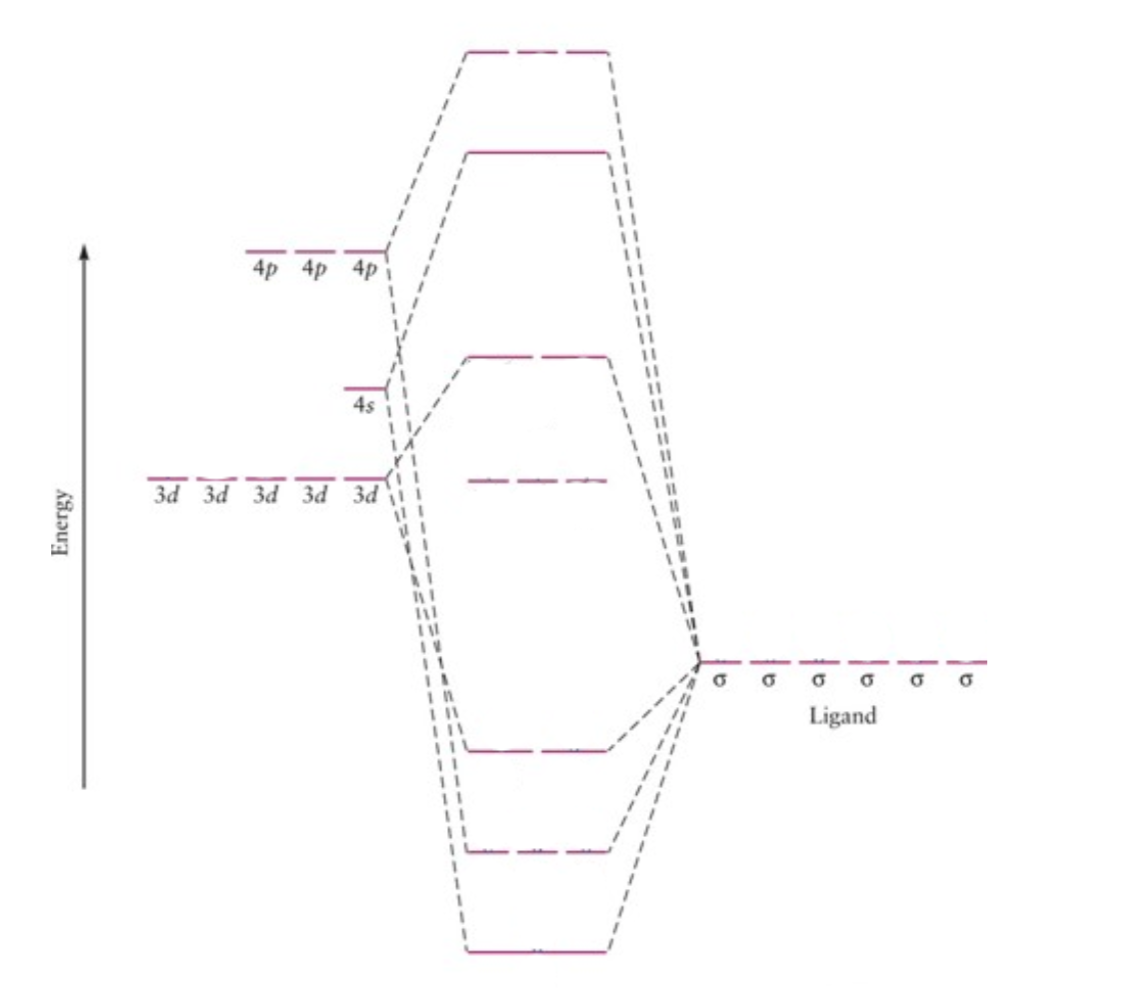
Construct a qualitative MO diagram for [Co(NH3)6]^3+, for which there is no π-bonding between the metal and the ligands. Label the diagram with the appropriate atomic orbitals for the metal, and labels the resulting MO's as either bonding, antibonding or non-bonding and add the correct number of electrons from the metal and ligands to the diagram.
The diagram showing orbital overlapping in the ammonia (NH3) molecule. The orbitals of NH3 participating in the bond formation to undergo sp3 hybridization . Molecular orbital diagram of ammonia (NH3) molecule. The molecular orbital diagram is a diagrammatic representation of how chemical bonding is taking place within the molecules.
The molecular orbital diagram of NH3 is presented in Figure 5 and will be elaborated in regards to its interactions. 4 MO theory and molecular geometry Walsh diagrams. They are totally symmetric to the operations of the C 3v point group. The Molecular Orbital Diagram for Ammonia Now it is time to draw the molecular orbitals of ammmonia and ...

Chemical Bonding Question A Draw An Orbital Overlap Diagram To Represent The Bonding In Ammonia Nh3 Homeworklib
* MO Energy Diagram for [Co(NH3)6]3+ If ligands are "lone pairs", with 6 lone pairs (octahedral) we always have 12 electrons from the ligands. Thus, the number of electrons in the "d-orbital" range of the MO = the number of electrons in the metal ion.
Ppt Ch2 Molecules And Covalent Bonding Lewis Structures Vsepr Mo Theory Powerpoint Presentation Id 1458708
Chem 302. The Molecular Orbitals of Ammonia. Determing the electronic structure of ammonia will introduce the new ideas of degenerate orbitals and degenerate axes. It is important to understand these concepts because of the large number of molecules that have point groups such as C 3v or D 3h. To determine the MO's of ammonia.
MO Theory • MO diagrams can be built from group orbitals and central atom orbitals by considering orbital symmetries and energies. • The symmetry of group orbitals is determined by reducing a reducible representation of the orbitals in question. This approach is used only when the group orbitals are not obvious by inspection.
Linear Combination Of Atomic Orbitals Wikipedia D Orbital Diagram Free Transparent Png Clipart Images Download
A further example is given using the NH3 MO diagram. Here, they calculate the bond order as 3, ignoring the fact that the NH3 a1 orbital is weakly bonding. If we were to in theory calculate the MO diagram for NH3 in a trigonal planar geometry (same as for BH3), we would also get the bond order as 3.
For the octahedral complex ion [CO(NH3)6]3+ a) Draw a molecular orbital (MO) energy level diagram. (2 Marks) b) Calculate the number of unparied electrons. (0.5 Mark) c) Indicate weather it is high spin or low spin complex. (0.5 Mark) 2. Breifly explain the synergic bond in the Cr(CO)6. Illustrate your answer with drawing, 12 Marks) (B) (5
An approximate MO diagram for the formation of [ML6 + (where M is a first row metal) using the ligand group orbital approach; the orbitals are shown pictorially in Figure 21.12. The bonding only involves M — L
To develop a MO scheme for NH3 assume that only the 2s and2p orbitals of nitrogen ... to the sigma bond between each carbon pair, the C–C bond order becomes.
A BRIEF INTRODUCTION TO MOLECULAR ORBITAL THEORY OF SIMPLE POLYATOMIC ... the polyatomic molecules H2O, NH3, BH3 and SiH4 using group theory is reported.
In contrast to crystal field theory, molecular orbital included the covalent nature of the metal-ligand bond interaction. No Metal- Ligand -bonding ( bonding only) Let's take [Co(NH3)6]3+ as an example. Using the LGO method, one can construct a qualitative MO diagram for bonding in a [ML6]n+ complex.
use qualitative MO theory to predict if BeH2 will be bent or linear. ... (do AFTER the problems class) draw a MO diagram for planar D3h NH3.
















0 Response to "37 mo diagram for nh3"
Post a Comment