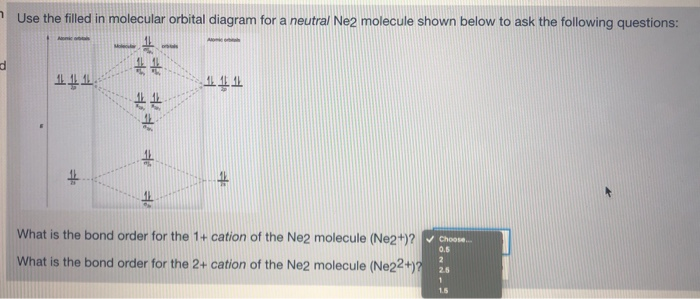
36 molecular orbital diagram for ne2 2+
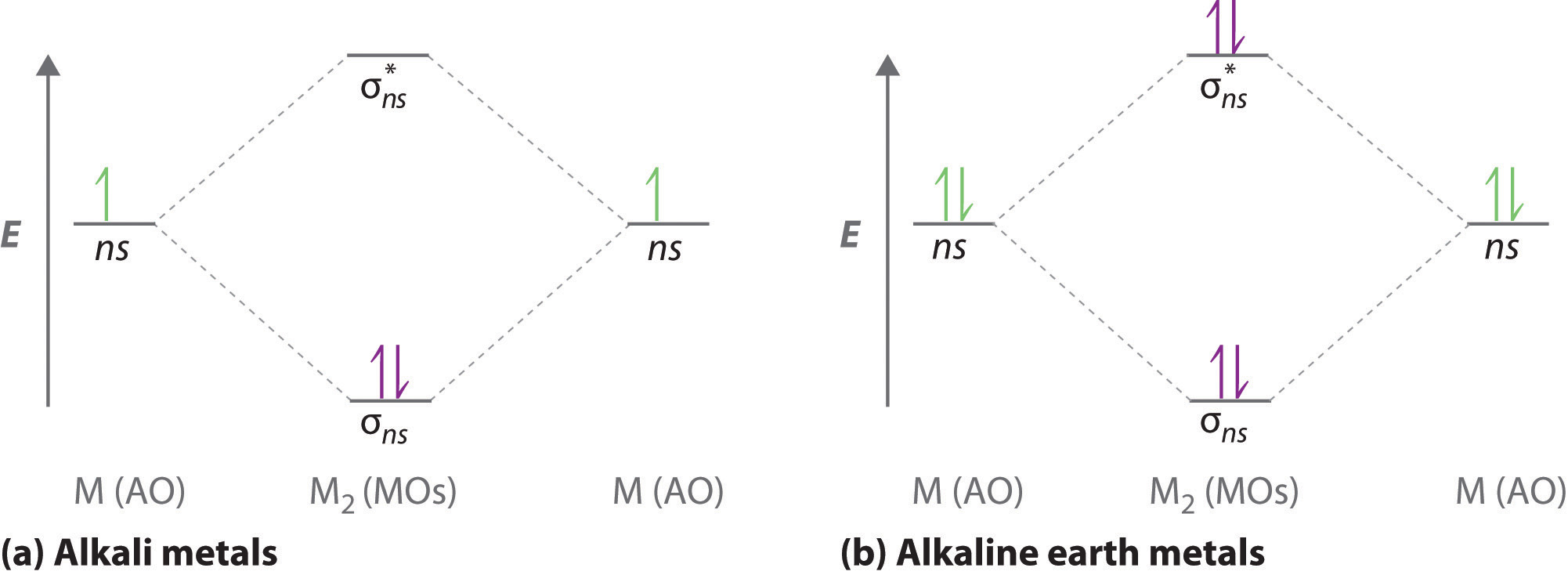
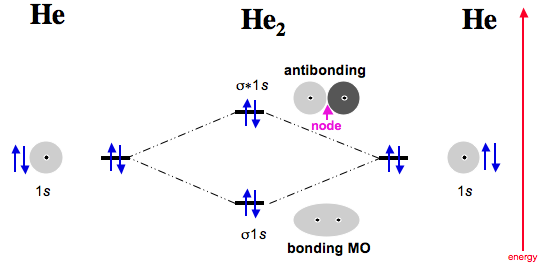
write the molecular orbital diagram of n2 and calculate ... An atomic orbital is monocentric while a molecular orbital is polycentric. Explain What is the relationship between bond order and the dissociation energy of a molecule? Out of H and H 2, which has higher first ionisation enthalpy? How are the shapes of molecular orbitals determined? He2 2+ Molecular Orbital Diagram - Wiring Diagrams He2+ MO diagram. Eg: Li + H; Li has 1s + 2s, while H has 1s. A molecular orbital explicitly describes the spatial distribution of a single electron orbitals, and σ∗. 1s is higher in energy. Draw this out using an energy level diagram: 2 He2 has bond order 0 [ (2 − 2)/2 = 0], and we can make H+. 2,. H−.
(PDF) Inorganic Chemistry by Miessler ~ 5th Edition ... This book is ideal for who want to use a strong molecular-orbital approach to explain structure and reactivity in inorganic chemistry. . × Close Log In. Log in with Facebook Log in with Google. or. Email. Password. Remember me on this computer. or reset password. Enter the email address you signed up with and we'll email you a reset link. ...
Molecular orbital diagram for ne2 2+
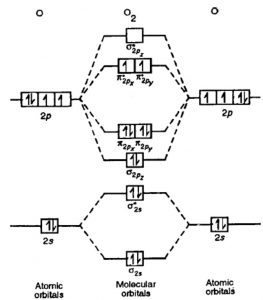
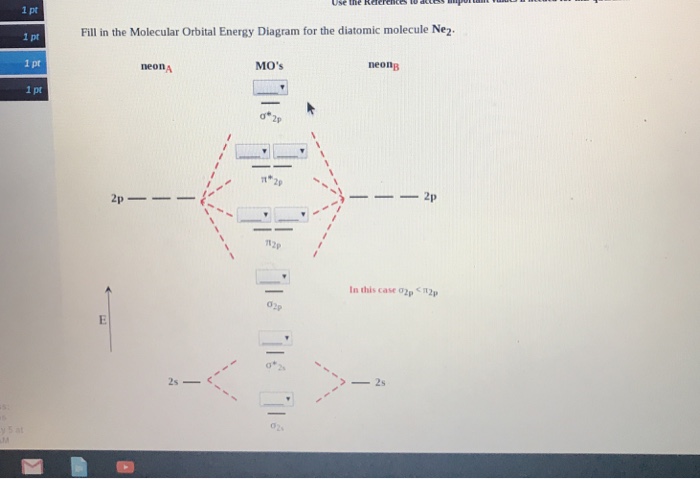
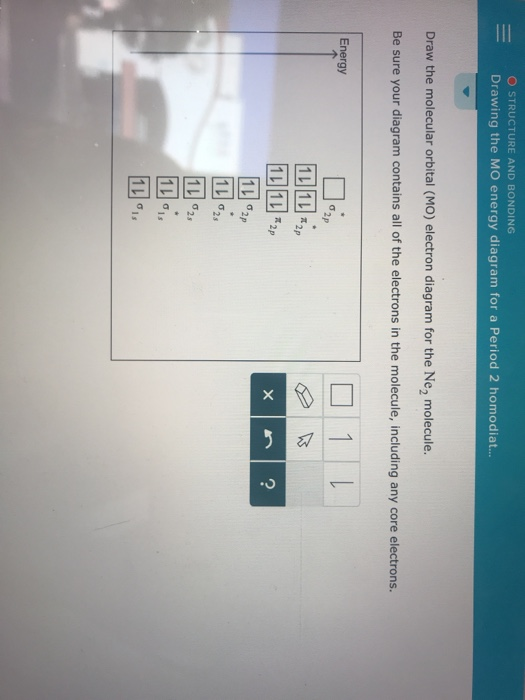
Draw the molecular orbital diagram of N2N2 + N2 Write ... Molecular orbital diagram of N 2 − is shown below: This picture shows the molecular orbital diagram of N 2 − . Orbitals represented by ∗ are antibonding orbitals and the orbitals without ∗ are bonding orbitals. Bond order can be calculated by the formula: Bond order = bonding electrons - antibonding electrons 2. Solved Draw the molecular orbital (MO) electron diagram ... Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. 90% (10 ratings) Transcribed image text: Draw the molecular orbital (MO) electron diagram for the Ne2 molecular ion. Be sure your diagram contains all of the electrons in the ion, including any core electrons. Is NE2 2+ paramagnetic or diamagnetic? - Quora Answer: Question: Is Ne2 2+ paramagnetic or diamagnetic? To understand this answer you have to know about molecular orbital (MO) theory of bonding. You can learn about it and its application to 2nd row elements here: The Central Science, Chapter 9, Section 8 Within that document is this diagram...
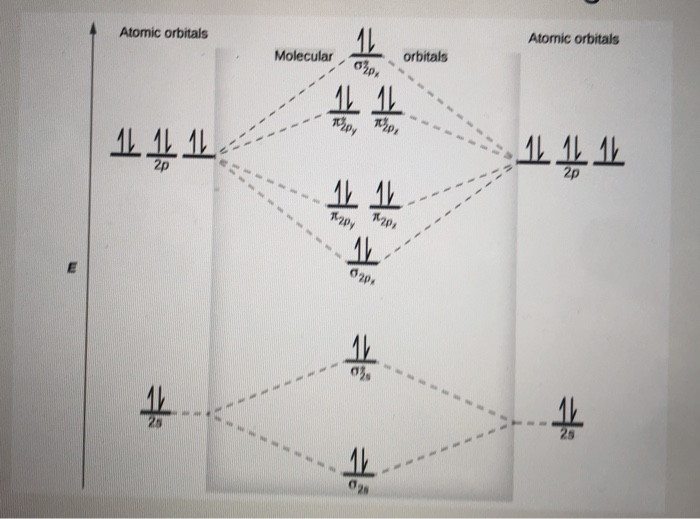
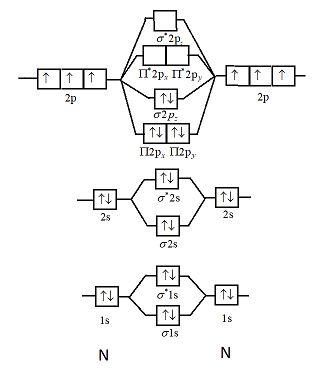
Molecular orbital diagram for ne2 2+. Draw a molecular orbital diagram of ${N_2}$ or ${O_2 ... Now, let us draw the molecular orbital diagram of ${N_2}$ . Now, first let us understand what magnetic behavior and bond order means. - Magnetic behavior: As we know the electron has an electron magnetic dipole moment, which is generally generated by the electron's spin property, which induces an electric charge into motion. As we can see the ... electronic configuration - Molecular orbital (MO) diagram ... Show activity on this post. I have been taught that the MO diagram is different for molecules with 14 or less electrons than the one used for molecules with 15 or more electrons. For N X 2 the orbitals in increasing energy are: σ 1 s < σ 1 s ∗ < σ 2 s < σ 2 s ∗ < π 2 p x, π 2 p y < σ 2 p z < π 2 p x ∗, π 2 p y ∗ < σ 2 p z ∗ ... Energy level diagram for Molecular orbitals - Chemical ... Energy level diagram for Molecular orbitals. ... If N b = Na,the molecule is again unstable because influence of electrons in the antibonding molecular orbital is greater than the bond influence of electron in the bonding molecular orbitals. 2) Stability of molecules in terms of bond order. Use the molecular orbital energy level diagram to show ... Click here👆to get an answer to your question ️ Use the molecular orbital energy level diagram to show that N2 would be expected to have a triple bond, F2 , a single bond and Ne2 , no bond.
Molecular Orbital Diagram For He2 A molecular orbital explicitly describes the spatial distribution of a single Energy Level Diagrams He2 has bond order 0 [ (2 − 2)/2 = 0], and we can make H+. According to the molecular orbital theory, in a supposed He2 molecule, both the if we draw its MOT DIAGRAM, 2 e's enter the Bonding molecular Orbital and 2 . NCERT Solutions for Class 11 Chemistry Chapter 4 ... Question 16. Why N 2 is more stable than O 2? Explain on the basis of molecular orbital theory. ... Draw the dipole diagram of H 2 O. Answer: The product of magnitude of charges (+ve, or -ve) and distance between them is called dipole moment. It is usually denoted by µ. Question 8. What are the main postulates of Valence Shell Electron Pair Repulsion (VSEPR) theory? Answer: … OneClass: ne2+ molecular orbital diagram Draw The Valence Bond Lewis Structure of Ne2^+2. Draw Molecular Orbital Diagram using Shorthand Notation. What is the bond order, number of sigma bonds, number of pi bonds? Is it paramagnetic? Which of the following are predicted by the molecular orbital model to be stable diatomic species? a. Solved Draw The Valence Bond Lewis Structure of Ne2^+2 ... Question: Draw The Valence Bond Lewis Structure of Ne2^+2. Draw Molecular Orbital Diagram using Shorthand Notation. What is the bond order, number of sigma bonds, number of pi bonds? Is it paramagnetic? This problem has been solved! See the answer See the answer See the answer done loading.
Chapter 11: Theories of Covalent Bonding Smartbook ... Using the attached molecular orbital energy diagram, select the correct molecular electron configuration for Ne2 2+. (Only molecular orbitals formed from valence atomic orbitals have been included.) A. (σ2s)2(σ∗2sσ2s )2(σ2p)2(π2p)2(π∗2pπ2p Molecular Orbital Diagram Ne2 - schematron.org Even rather simple molecular orbital (MO) theory can be used to predict from the bottom of the diagram because this is how MO diagrams are constructed, from N2, O2, F2, Ne2 the complexity of the molecular orbitals develop in two ways. Page 1. MO Diagrams for Elements Li2 through Ne2. (Don't memorize.) Li2 through N2. O2 through Ne2. Chemistry II Kinetics Test Flashcards - Quizlet From Molecular Orbital Diagram, which is most stable? A. F2 2-B. Ne2 2+ C. O2 2+ D. F2 E. F2 2+ C. O2 2+ Choose the compound below that should have the highest melting point according to the ionic bonding model. A. CaS B. NaCl C. RbI D. MgO E. AlN. E. AlN. With the help of molecular orbital diagram show that Ne2 ... With the help of molecular orbital diagram show that Ne2 cannot exist as stable molecule. (Atomic number of Ne = 10).
MO Diagram for N2+ (Molecular Orbital) - YouTube There are two MO diagrams you need to memorize for diatoms (N2, O2, Ne2, etc).One is for the elements up to Nitrogen. The other is for AFTER nitrogen (start...
MO Diagram for N2+ (Molecular Orbital) lesson plan | Spiral Interactive video lesson plan for: MO Diagram for N2+ (Molecular Orbital) Activity overview: There are two MO diagrams you need to memorize for diatoms (N2, O2, Ne2, etc).
Molecular Orbital Diagram For Ne2 - Wiring Diagram Pictures Even rather simple molecular orbital (MO) theory can be used to predict from the bottom of the diagram because this is how MO diagrams are constructed, from N2, O2, F2, Ne2 the complexity of the molecular orbitals develop in two ways.Draw the molecular orbital diagram for Ne 2 + and determine if the bond between the two atoms will be stable.
Recitation Week 10 (test 3 - Recitation 2) - GitHub Pages A) O2^2-B) Ne2^2+ C) O2^2+ D) F2^2+ E) None of the above are paramagnetic; 3) Draw the molecular orbital diagram needed, and determine which of the following is paramagnetic. A) B2^2+ B) B2^2-C) N2^2+ D) C2^2-E) B2; 4) Draw the molecular orbital diagram shown to determine which of the following is most stable.
Why does the Ne2 molecule not exist using molecular ... Answer (1 of 4): Molecular orbital confuguration of Ne2 is σ1s²σ*1s²σ2s²σ*2s²σ2Pz²π2Px²π2Py²π*2Px²π*2Py²σ*2Pz² Hence the bond order of Ne2 according to M.O.T is =(Nb-Na)/2 =(10-10)/2 (since,both bonding orbitals and non-bonding orbital contains 10 electrons) =0 Hence, no bond is possible b...
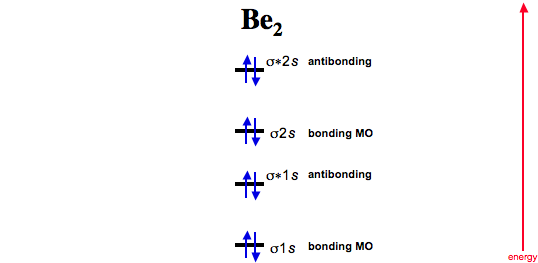
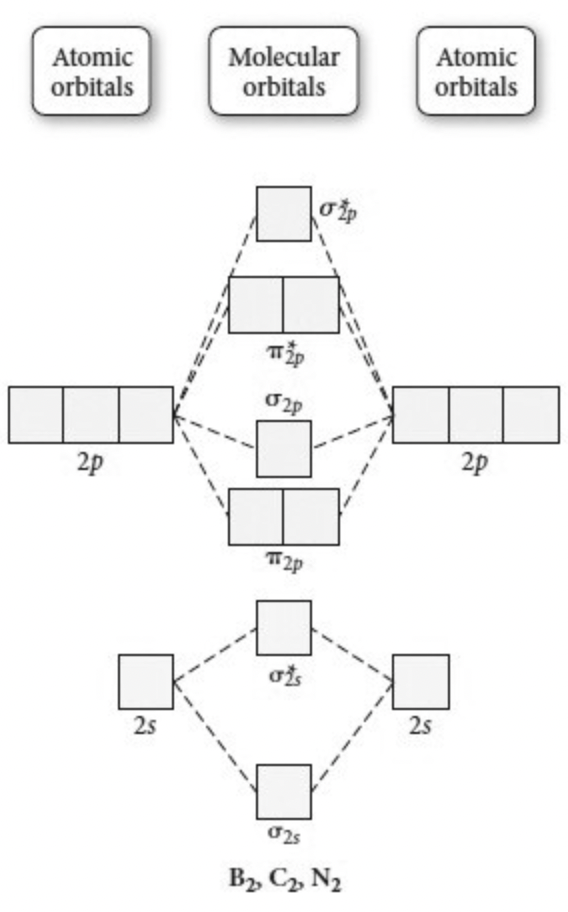
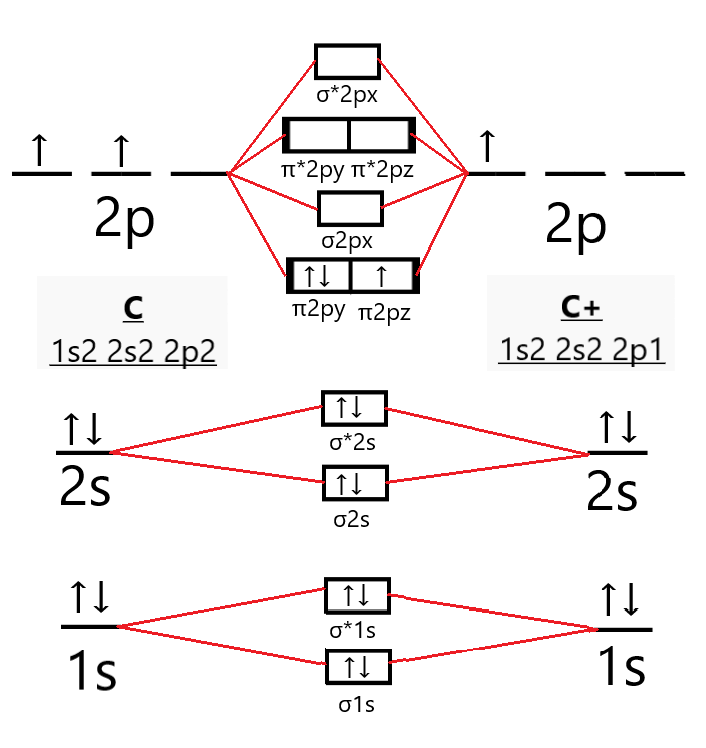
What is the molecular orbital diagram for B_2? | Socratic Before we can draw a molecular orbital diagram for B₂, we must find the in-phase and out-of-phase overlap combinations for boron's atomic orbitals. Then we rank them in order of increasing energy. We can ignore the 1s orbitals, because they do not contain the valence electrons. Each boron atom has one 2s and three 2p valence orbitals. The 2s orbitals will overlap to form 2sσ and 2sσ ...
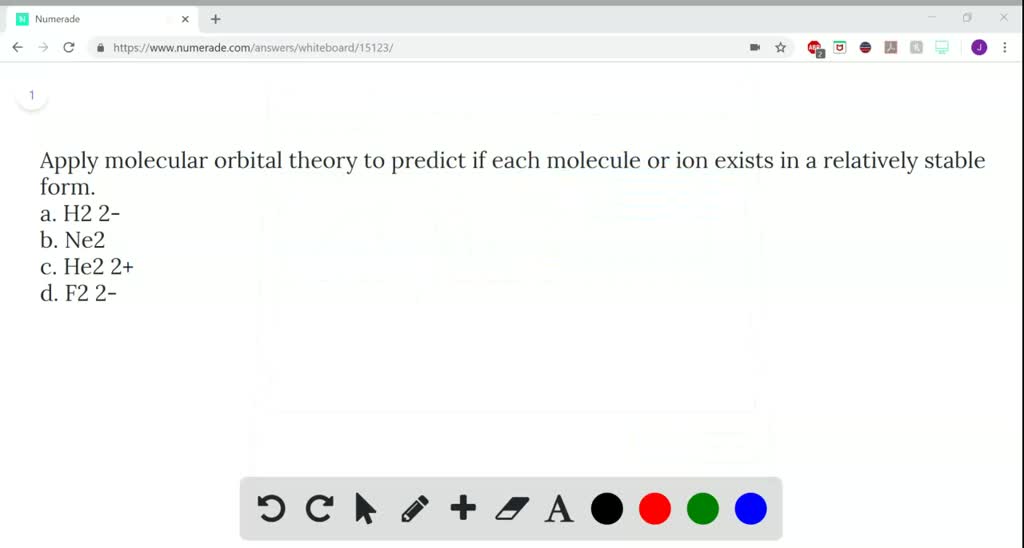
apply molecular orbital theory to predict if each molecule or ion exists in a relatively stable form
with the help of molecular orbital theory show that ne2 ... According to Molecular Orbital theory, only those molecule can exists which have net positive bond order while the molecules with negative or zero bond order will not exists. In case of Ne 2 molecule, since, Ne atom has 10 electrons so total electrons are 20 the configuration is given as:
What is the molecular orbital diagram for C_2^-? | Socratic The lowest energy unoccupied molecular orbital is 2pσ, so that is where the extra electron will be added. The electron configuration of the neutral C2 molecule is -- I'll use the notation given to you in the diagram. C2:(1sσ)2(1s* σ)2(2sσ)2(2s* σ)2(2pπ)4. The electron configuration of the C− 2 ion will be.
Chemistry the central science 14th edition - Academia.edu Academia.edu is a platform for academics to share research papers.
Molecular Orbital MO Diagram for N2(2-) - YouTube the pi(2p) bonding orbitals are LOWER than the sigma(2p) bonding orbitals.N2(2-) has a bonding order of 2, which predicts that there will be a stable double ...
Molecular orbital diagram - Wikipedia A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals combine to form the same number of ...
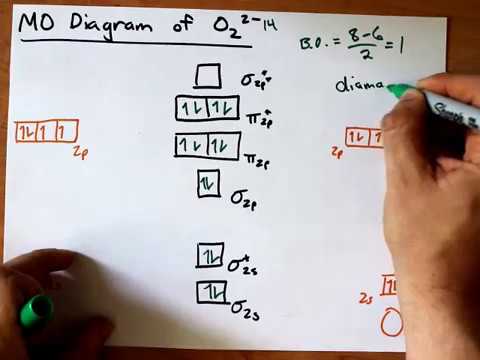
PDF B di II:Bonding II: Molecular Geometry and Bonding Theories Molecular Orbital Diagram of O 2 Chapter 9 Section 6 When filling the MO levels, you have to: Count the number of valence electrons, Start with the lower energy orbitals first, Follow Hund's rule, and Pt t th t Dr. A. Al-Saadi 19 Put not more than two electrons in one MO. Molecular Orbital Diagram of N 2 Chapter 9 Section 6
Molecular Orbital Diagram For Ne2 Mar 4, Find an answer to your question Draw and explain the molecular orbital diagram of Ne2. On the basis of molecular orbital diagram, explain. According to Molecular Orbital theory, only those molecule can exists which have net positive bond order while the molecules with negative or.
Is NE2 2+ paramagnetic or diamagnetic? - Quora Answer: Question: Is Ne2 2+ paramagnetic or diamagnetic? To understand this answer you have to know about molecular orbital (MO) theory of bonding. You can learn about it and its application to 2nd row elements here: The Central Science, Chapter 9, Section 8 Within that document is this diagram...
Solved Draw the molecular orbital (MO) electron diagram ... Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. 90% (10 ratings) Transcribed image text: Draw the molecular orbital (MO) electron diagram for the Ne2 molecular ion. Be sure your diagram contains all of the electrons in the ion, including any core electrons.
Draw the molecular orbital diagram of N2N2 + N2 Write ... Molecular orbital diagram of N 2 − is shown below: This picture shows the molecular orbital diagram of N 2 − . Orbitals represented by ∗ are antibonding orbitals and the orbitals without ∗ are bonding orbitals. Bond order can be calculated by the formula: Bond order = bonding electrons - antibonding electrons 2.















0 Response to "36 molecular orbital diagram for ne2 2+"
Post a Comment