39 reaction coordinate diagram endothermic vs exothermic
Reaction profiles - Exothermic and endothermic reactions - AQA... Learn about exothermic and endothermic reactions and the transfer of energy with GCSE Bitesize Chemistry (AQA). An energy level diagram shows whether a reaction is exothermic or endothermic. It shows the energy in the reactants and products, and the difference in energy... Endothermic vs. Exothermic Endothermic vs. Exothermic - - - Difference between Endothermic and Exothermic. A quick difference between endothermic and exothermic involves reactions in the environment. An endothermic reaction takes place when energy is absorbed from surroundings in the form of heat...
Difference Between Endothermic Reactions and Exothermic... Endothermic Reactions vs. Exothermic Reactions. Exothermic Reactions. A reaction in which system absorbs energy from the environment is called endothermic reaction.

Reaction coordinate diagram endothermic vs exothermic
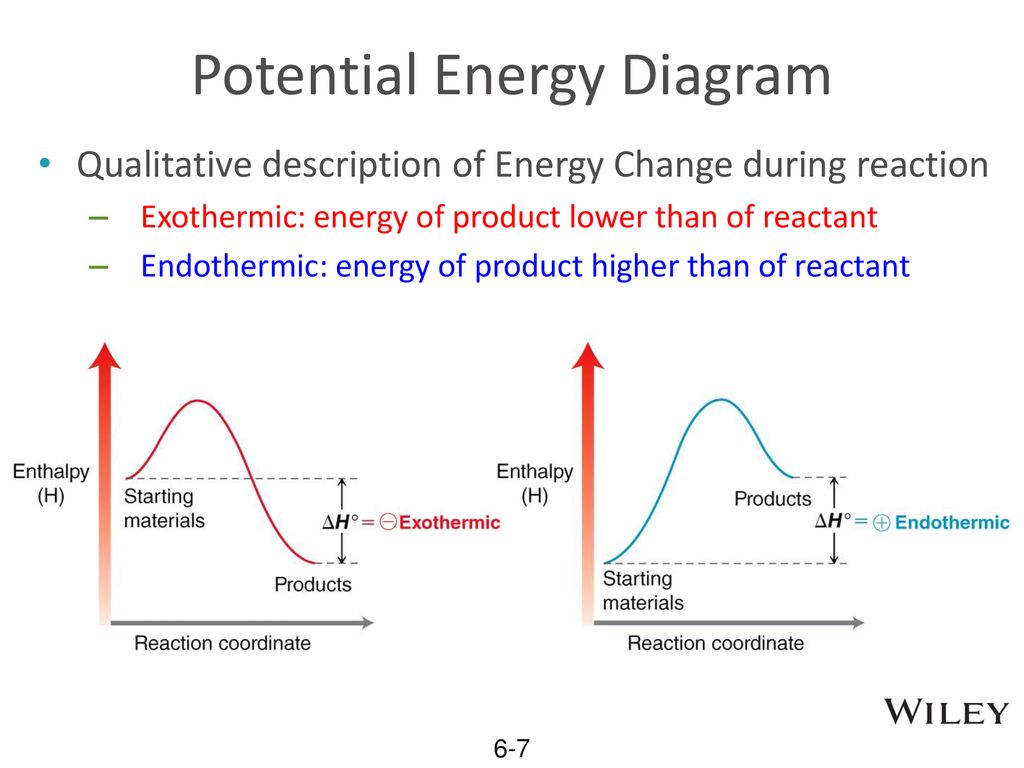
Difference between Endothermic and Exothermic Reactions These reactions are the opposite of an endothermic reaction. It will release the energy by light or heat into the surrounding. So, exothermic reactions are involving the release of energy. These types of reactions are warmer, and sometimes they may be dangerous with high rate reactions. Lesson 1: Exothermic and endothermic reactions Energy level diagrams for exothermic reactions In an exothermic reaction, reactants have a higher energy level than the products. Is this reaction endothermic or exothermic? 2P + 5Cl2 2PCl5 ΔHo = -886 kJ 5. How much heat will be released when 4.77 grams of ethanol (C2H5OH) reacts with... Endothermic vs. exothermic reactions (article) | Khan Academy Endothermic vs. exothermic reactions. This is the currently selected item.
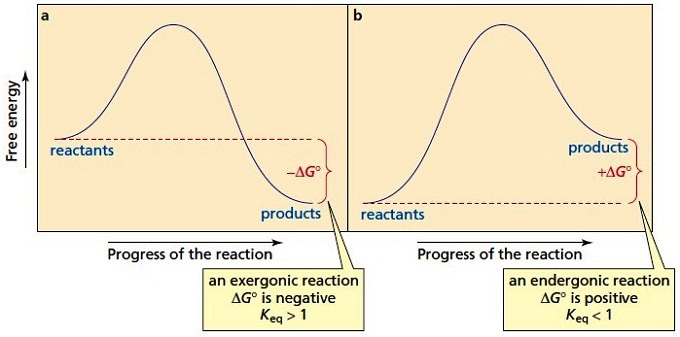
Reaction coordinate diagram endothermic vs exothermic. Endothermic vs. Exothermic Reactions Endothermic vs. Exothermic Reactions. FAQs. What is Endothermic Reaction? It is possible to represent the endothermic reaction in the form of a graph called the energy diagram. The potential energy of the reaction is plotted along the vertical axis, and time is plotted along the horizontal axis. Endothermic Vs. Exothermic Reactions - Science Struck Endothermic Vs. Exothermic Reactions: Comparison. Every change that you see in a natural system involves the transaction of energy. During an exothermic reaction, entropy increases substantially due to the heat released. Enthalpy decreases when the reaction occurs at constant pressure, while... Endothermic and Exothermic Chemical Reactions Endothermic vs Exothermic Comparison. Endergonic and Exergonic Reactions. Resources and Further Reading. Endothermic and exothermic reactions refer to the absorption or release of heat. There are other types of energy which may be produced or absorbed by a chemical reaction. Exothermic vs. Endothermic and K - Chemistry LibreTexts An exothermic reaction occurs when the temperature of a system increases due to the evolution of heat. The enthalpies of these reactions are less than zero, and are therefore exothermic reactions. A system of reactants that absorbs heat from the surroundings in an endothermic reaction has a...
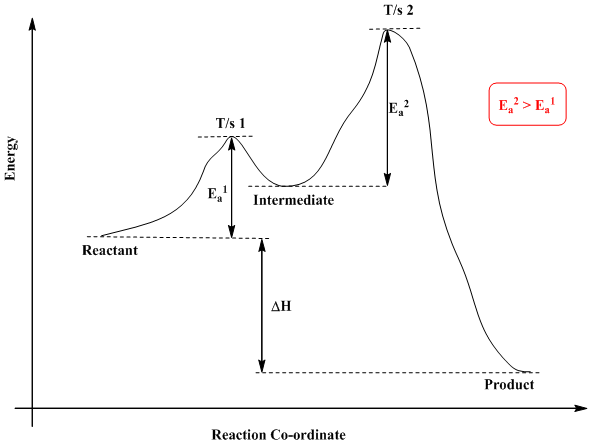
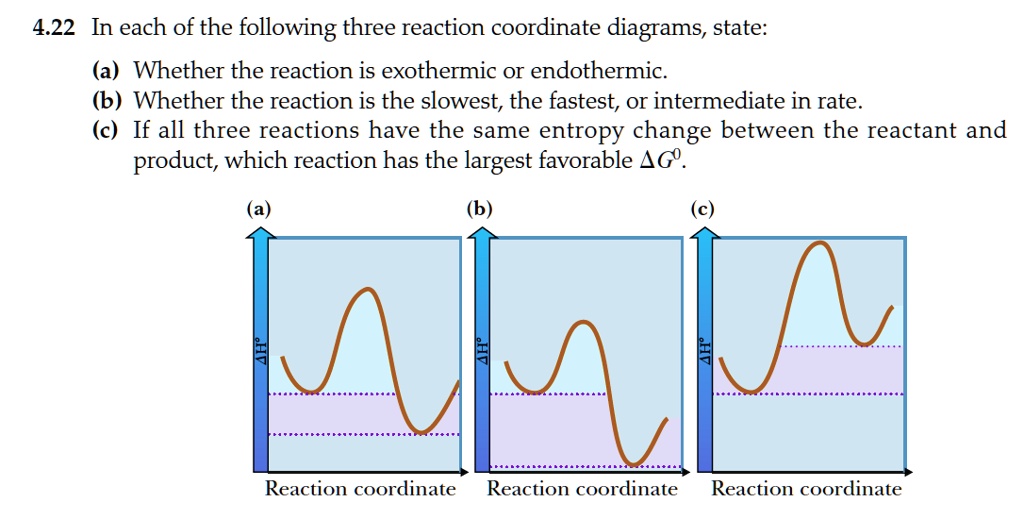
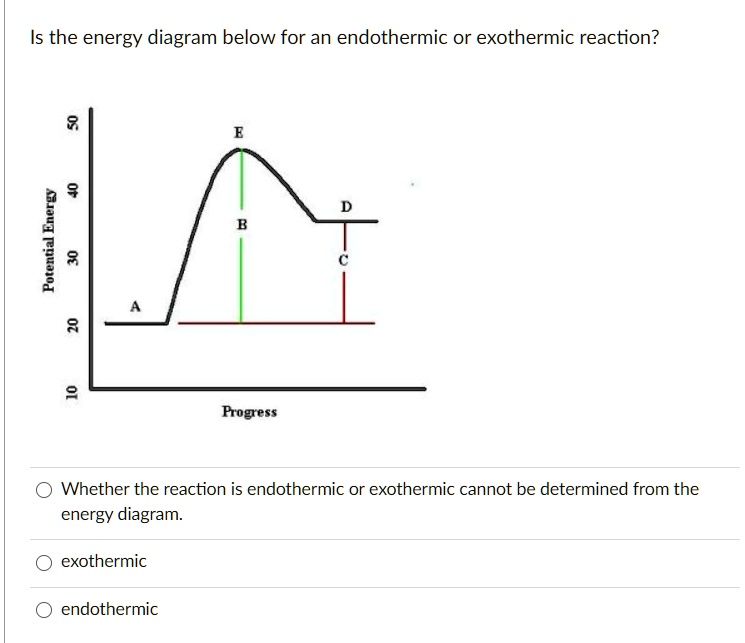
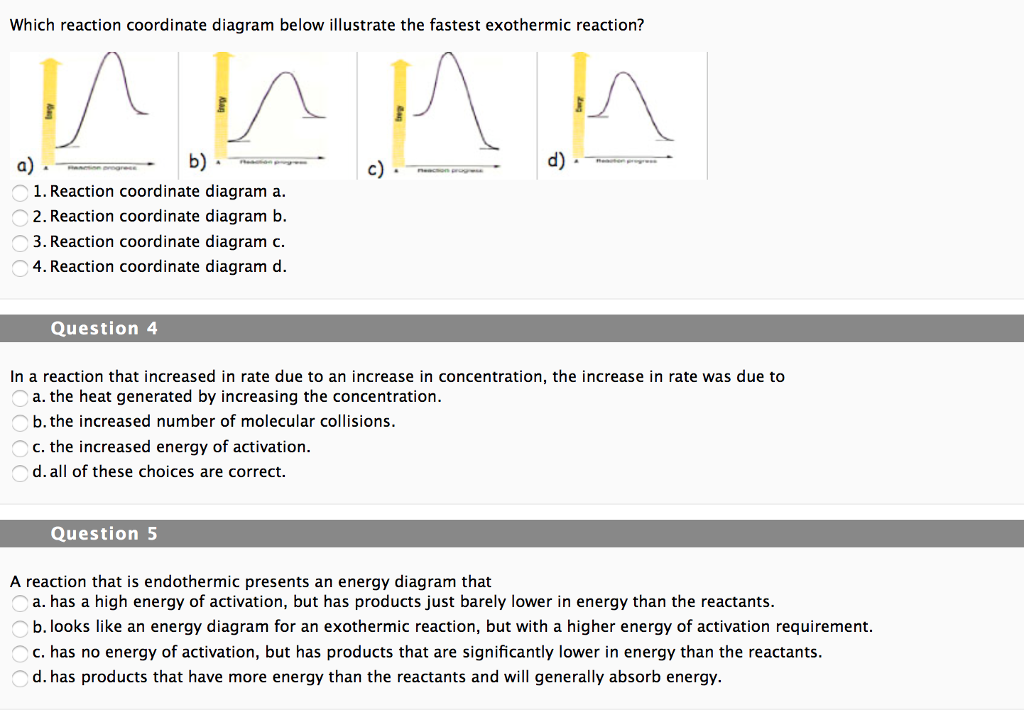
Difference between Endothermic and Exothermic Reaction Endothermic Vs. Exothermic Reaction. What is the difference between endothermic and exothermic reactions? Well, When the molecules interact with each other several reactions take place. Exothermic and Endothermic Processes | Introduction to Chemistry Endothermic and exothermic reactionsPaul Andersen explains how heat can be absorbed in endothermic or released in exothermic reactions. An energy diagram can be used to show energy movements in these reactions and temperature can be used to measure them macroscopically. Key Differences Between Endothermic and Exothermic Reaction Content: Endothermic Vs Exothermic Reaction. Comparison Chart. Enthalpy change of the reaction. What is an Endothermic Reaction? ∆H = H Product - H Reactant. Exothermic Reaction: Heat released: 0 > ∆H, i.e., Negative. Endothermic Reaction: Heat Absorbed: 0 < ∆H, i.e., Positive. Exothermic or Endothermic reactions - Chemistry Stack Exchange Can we determine whether a reaction is endothermic or exothermic? For example if we are given the following reactions Can we determine weather these reactions are endothermic or exothermic or do we have to just memorize this? Are there any general trends that could make memorizing this...
Endothermic Vs. Exothermic Reaction Graphs - YouTube Endothermic Vs. Exothermic Reaction Graphs. Смотреть позже. exothermic vs endothermic and why | Gold Refining & Metal... An exothermic reaction is a reaction that builds heat.The greater the reaction and the more vigorous the reaction is,the hotter the solution will become.Exothermic reactions occur mainly when you are lowering the PH of a solution.Normally,when you dissolve a metal into a solution,it will build heat but... Endothermic vs Exothermic Reactions | ChemTalk Endothermic vs Exothermic Reactions. February 2, 2022. Posted by Sam Birrer. You can think about this visually using a reaction energy diagram, as seen below Endothermic and exothermic reactions are everywhere, even when we don't notice the change in temperature they create. Examples of Endothermic and Exothermic Reactions | Energy and... There are many examples of endothermic and exothermic reactions that occur around us all the time. Physical changes can also be classified as exothermic or endothermic. When we are referring to physical change then we talk about exothermic or endothermic processes.
Similarities between Exothermic and Endothermic Reactions Learn the difference between Exothermic and endothermic reaction. The basis of comparison include: description, production, change in enthalpy, end-product stability Exothermic reactions are reactions or processes that releases energy, usually in the form of heat or light to its environment.
Difference Between Endothermic and Exothermic Reactions What are Exothermic Reactions? An exothermic reaction is a process that releases energy to the surroundings, usually in the form of heat. Summary - Endothermic vs Exothermic Reactions. Endothermic and exothermic are terms related to heat transfer in thermodynamic systems.
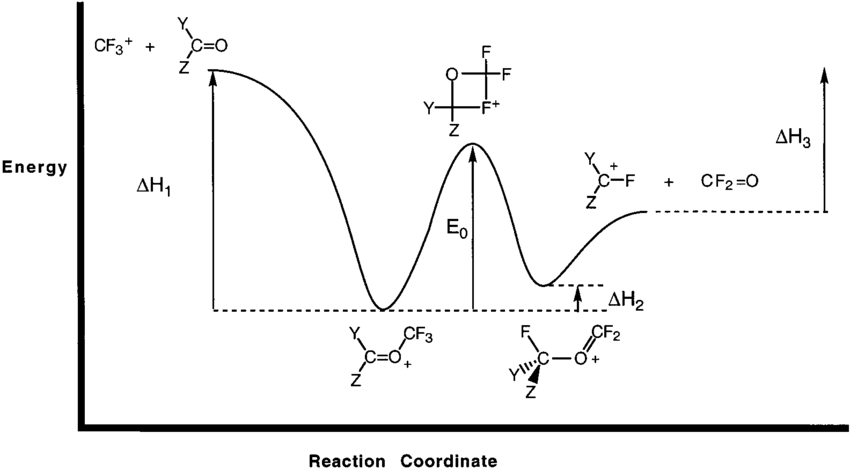
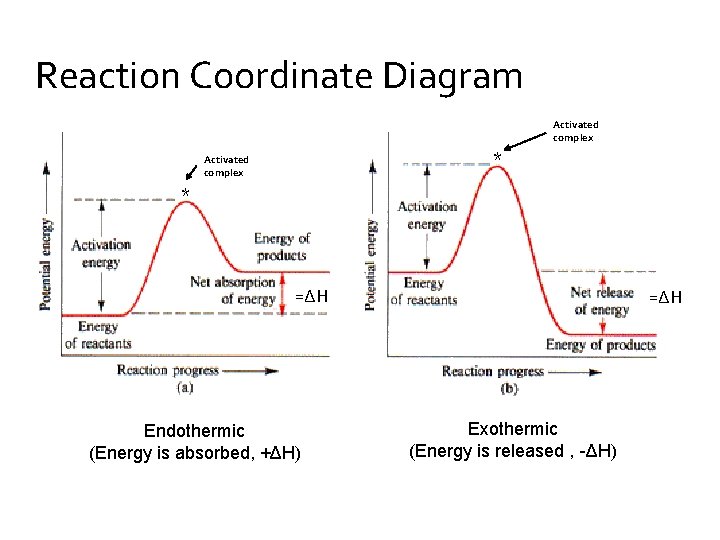
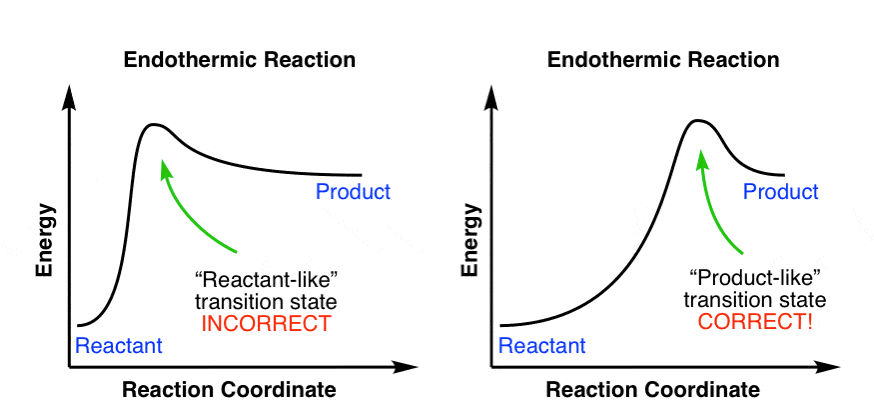
Enthalpy change of a reaction, Exothermic and Endothermic... Endothermic vs Exothermic reactions. Bond breaking vs Bond making. The Mechanics of Exothermic and Endothermic Reactions. In an exothermic reaction, the energy released during Enthalpy level diagrams: For Exothermic reaction, ΔH is negative, which means the system...
Difference between Exothermic and Endothermic Reactions These reactions are exothermic reactions and endothermic reactions. What is an Exothermic Reaction? Endothermic Reactions- The term "endothermic reaction" refers to a process in which a system absorbs energy in the form of heat from its surroundings.
Difference Between Endothermic and Exothermic Reactions Main Difference - Endothermic vs Exothermic Reactions. Chemical reactions can be divided into two groups as endothermic reactions and exothermic reactions according to the energy transfer between the surrounding and the system where the reaction is taking place.
Diagram of Endothermic VS Exothermic Reactions... | Quizlet Endothermic VS Exothermic Reactions, 6.1 - Collision Theory, PE diagram. STUDY. endothermic. dry ice forming "fog" at a Halloween party-HEAT ENTERS DRY ICE TO MAKE FOG. exothermic. magnesium + oxygen-HEAT IS RELEASED DURING THE REACTION.
Endothermic and Exothermic Reactions Lab iTeachly.com Endothermic vs Exothermic Reactions Activity. Develop a model to illustrate that the release or Draw an energy profile diagram for the reaction between ethanoic acid and sodium carbonate. What is the Difference between Exothermic and Endothermic Reactions? Here is a good video to show...
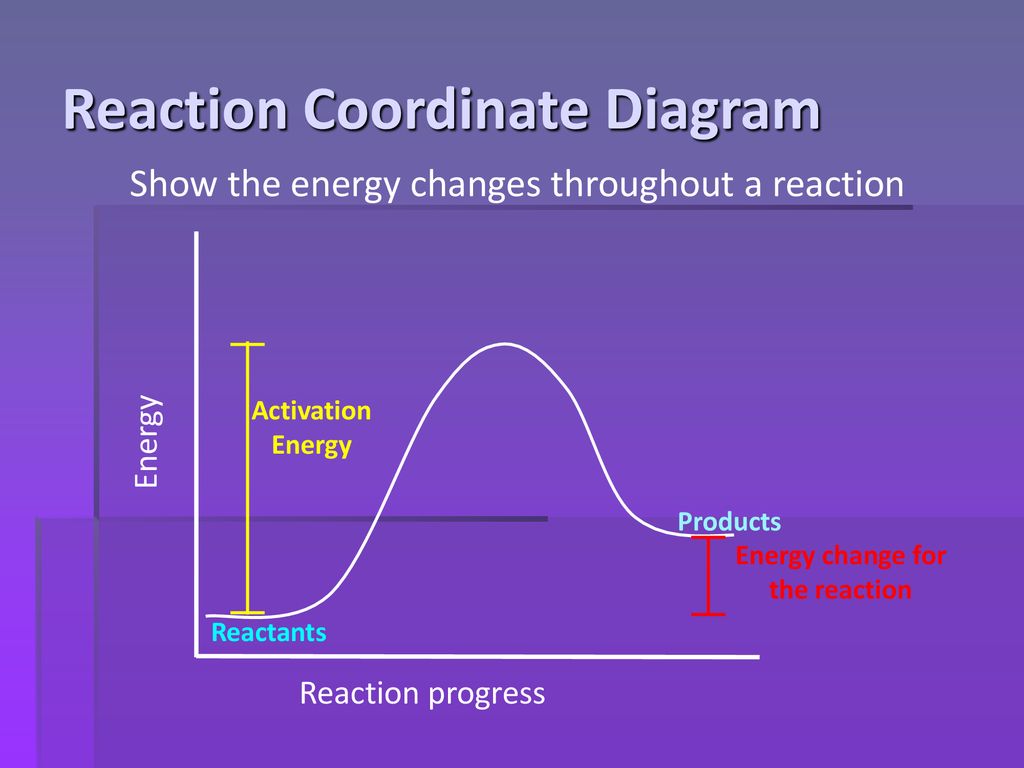
Energy profile diagrams for endothermic and exothermic reactions In an exothermic reaction, the reactants lose heat energy to form products. Thus, the products formed have less energy than the reactants, Hproducts < Hreactants. Figure shows the energy level diagram of an endothermic reaction. The following are the steps to construct energy level diagrams for...
Endothermic and Exothermic Reactions - Vernier Endothermic and Exothermic Reactions. Experiment #1 from Chemistry with Vernier. Some chemical reactions absorb energy and are called endothermic reactions. You will study one exothermic and one endothermic reaction in this experiment.
Endothermic vs Exothermic Reactions - Difference and... | Diffen What's the difference between Endothermic and Exothermic? An endothermic reaction occurs when energy is absorbed from the surroundings in the form of heat. Conversely, an exothermic reaction is one in which energy is released from the system into the surroundings. The terms are commonly used...
Exothermic and Endothermic Reactions - ppt video online download 4 Endothermic and exothermic reactions Step 1: Energy must be SUPPLIED to break chemical bonds EXOTHERMIC REACTIONS The PE diagram goes downhill. 8 Endothermic The sum of the 12 Measuring Heat reaction Exothermic reaction: heat given off & temperature of water rises...
Exothermic vs. Endothermic Reaction | Forum How do we know if a reaction is endothermic or exothermic? Can we only know if we are given delta H? I don't think you can tell whether a reaction is exothermic or endothermic by the equation alone.
Endothermic vs. exothermic reactions (article) | Khan Academy Endothermic vs. exothermic reactions. This is the currently selected item.
Lesson 1: Exothermic and endothermic reactions Energy level diagrams for exothermic reactions In an exothermic reaction, reactants have a higher energy level than the products. Is this reaction endothermic or exothermic? 2P + 5Cl2 2PCl5 ΔHo = -886 kJ 5. How much heat will be released when 4.77 grams of ethanol (C2H5OH) reacts with...
Difference between Endothermic and Exothermic Reactions These reactions are the opposite of an endothermic reaction. It will release the energy by light or heat into the surrounding. So, exothermic reactions are involving the release of energy. These types of reactions are warmer, and sometimes they may be dangerous with high rate reactions.








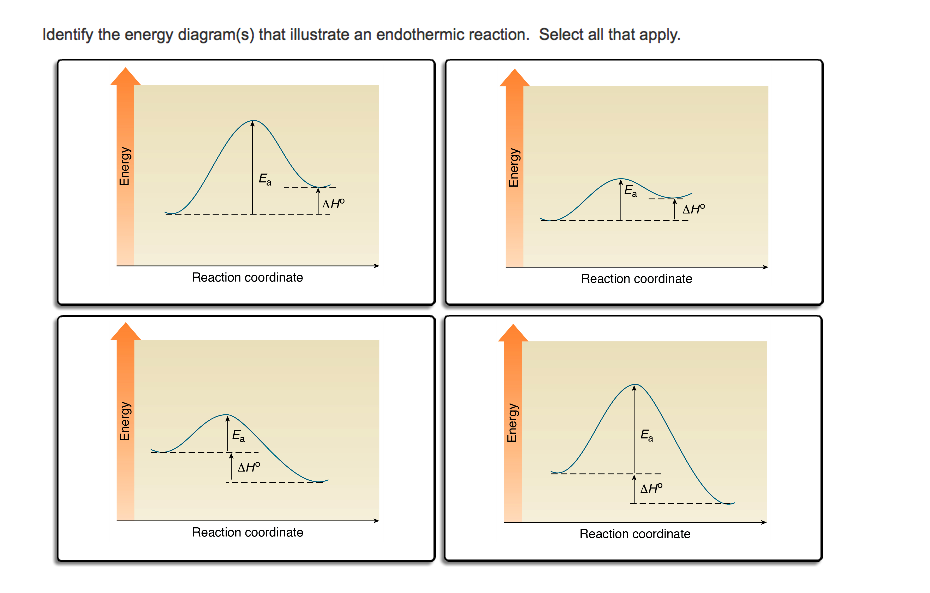




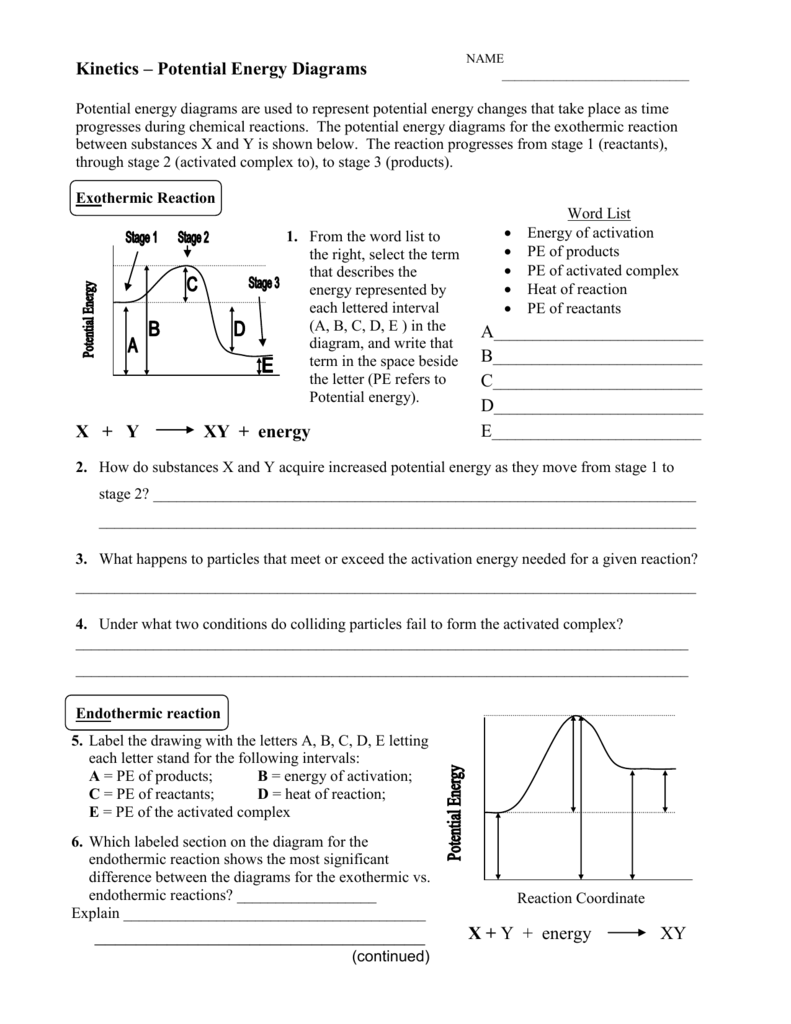

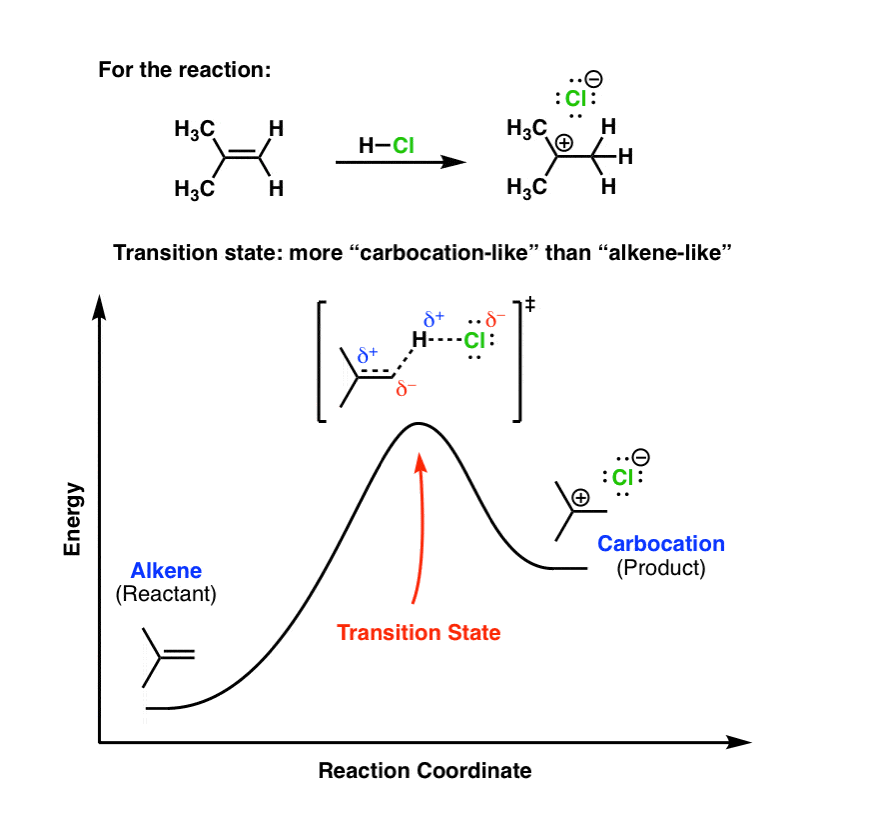
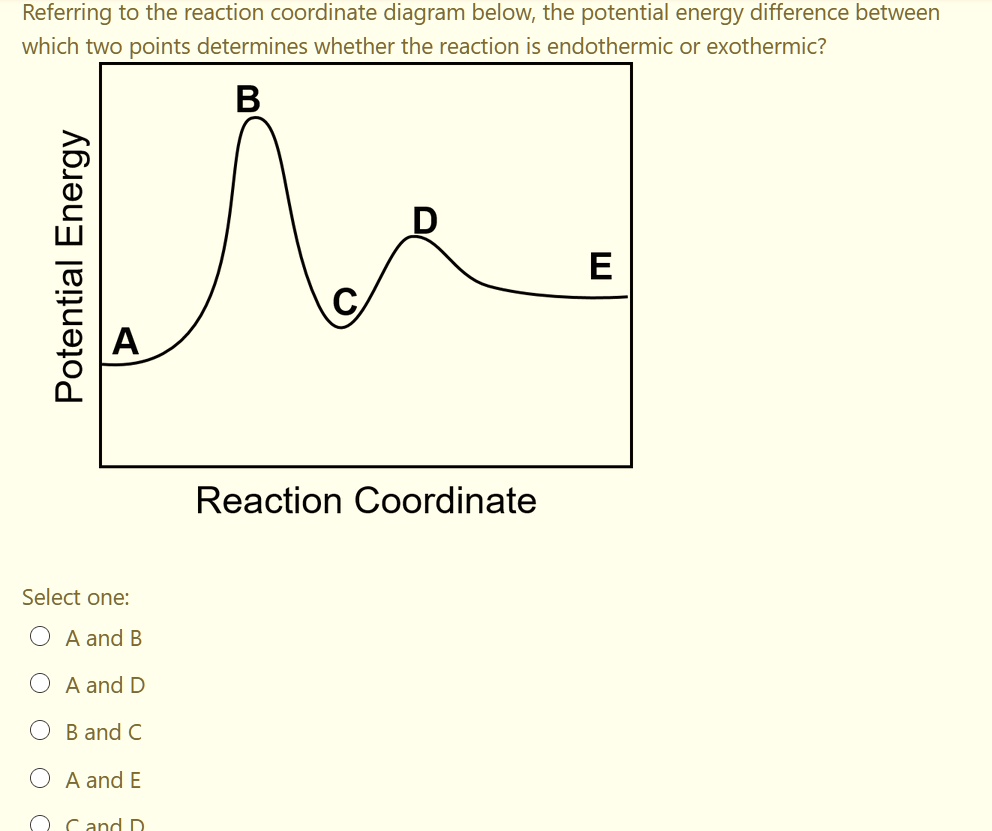




0 Response to "39 reaction coordinate diagram endothermic vs exothermic"
Post a Comment