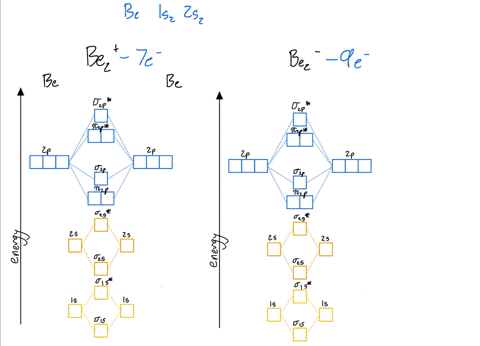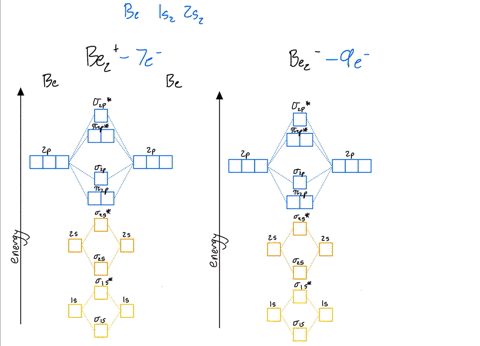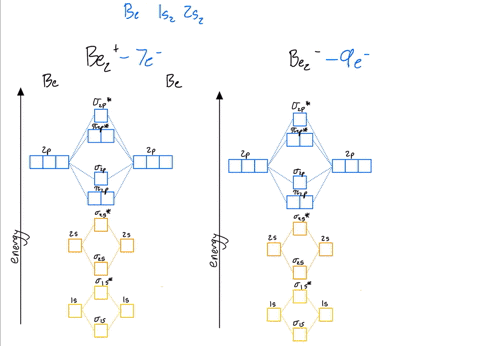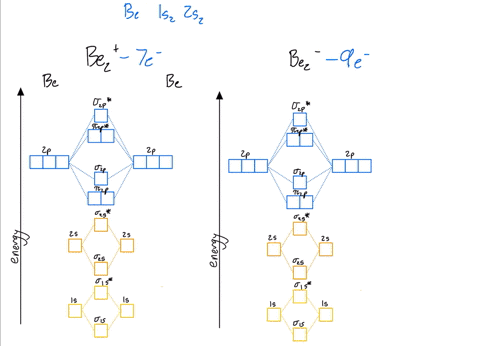
39 be2+ molecular orbital diagram
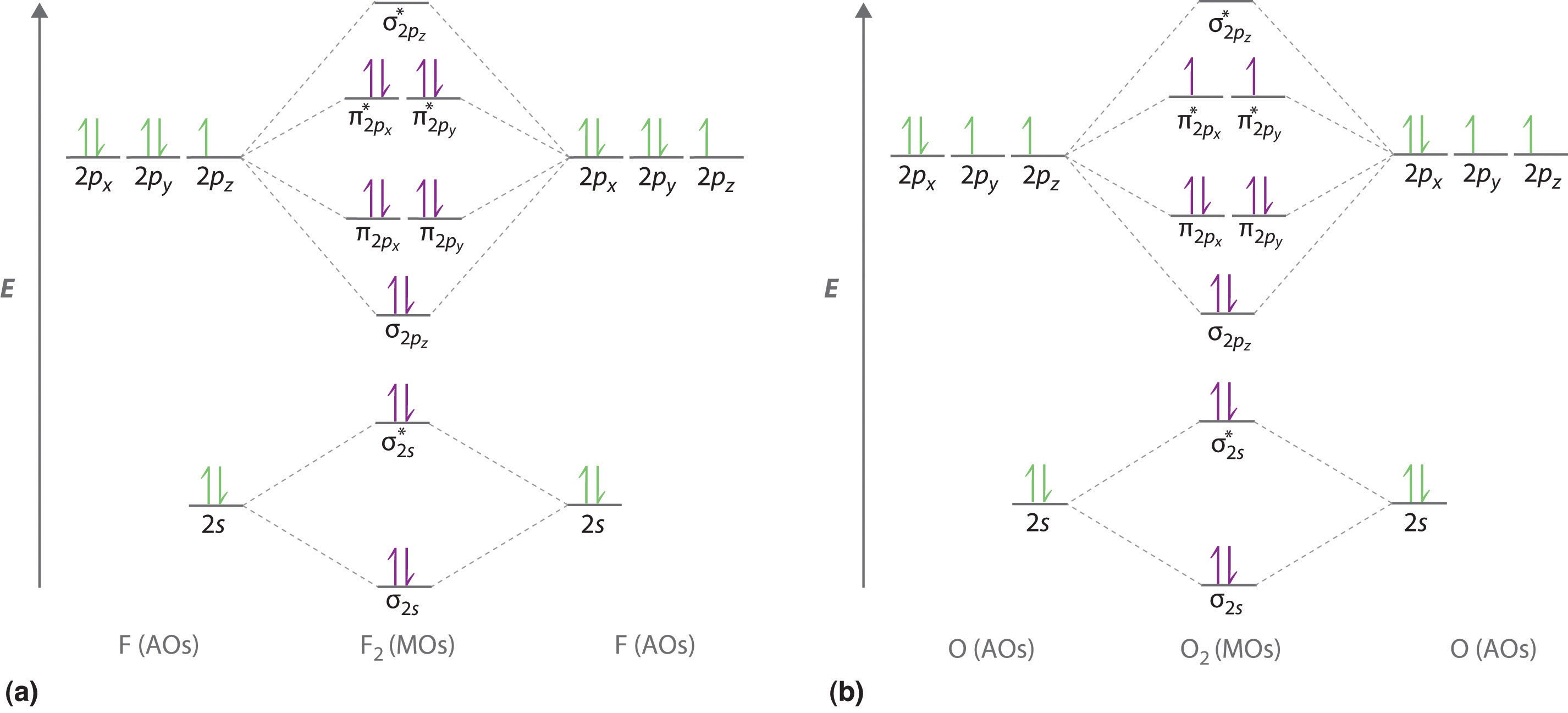
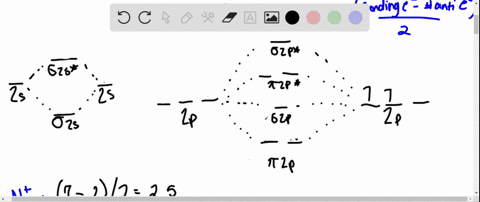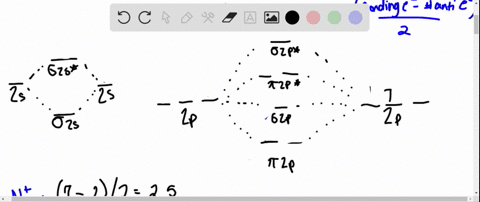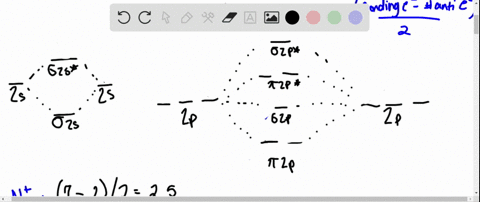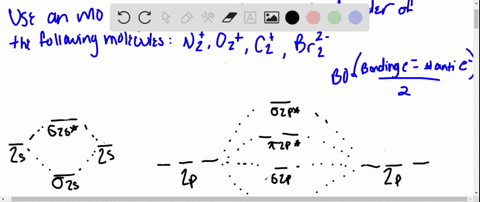
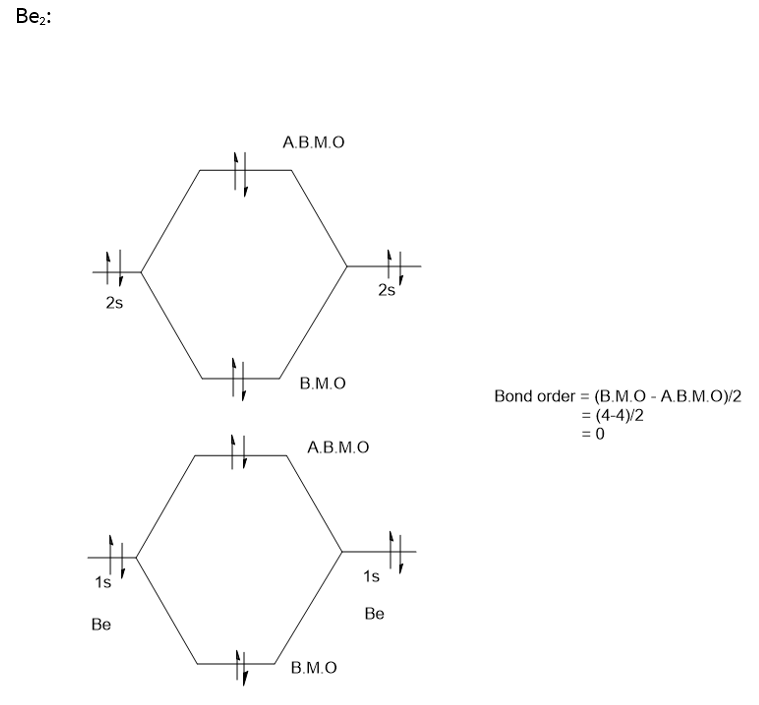
Draw the molecular orbital diagram for:(i) Be2(ii) B2 and ... (i) Be2 molecule: The electronic configuration of Be(Z = 4) is: 4 Be 1s 2 2s 1 Be 2 molecule is formed by the overlap of atomic orbitals of both beryllium atoms. Number of valence electrons in Be atom = 2 Thus in the formation of Be 2 molecule, two outer electrons of each Be atom i.e. 4 in all, have to be accommodated in various molecular orbitals in the increasing order of their energies. Solved #15. The Molecular Orbital diagram below is | Chegg.com The Molecular Orbital diagram below is appropriate for Li2 and Be2. After completing the diagram for Li2 and Bez, determine which of the following statement is true. 02p* Izp* 62P IZP 023* 023 013* Ols A) both are stable and diamagnetic. B) Li2 is stable and diamagnetic, but Bez is unstable. C) Bez is stable and diamagnetic, but Liz is unstable.
40 molecular orbital diagram for he2+ - Diagram For You So the bond order of B2 is equal to 1, which you can get by drawing the molecular orbital diagram and performing the equation Bond Order = . 5 * (# of bonding electrons - # of antibonding electrons). However, when you draw the Lewis structure of B2, you get a triple bond . On the basis of molecular orbital theory, explain why `He ...
Be2+ molecular orbital diagram
34 Be2 Mo Diagram Wiring Diagram Database - Dubai Burj ... How To Make The Molecular Orbital Diagram For Be2: Does The Molecule Exist? this video discusses how to draw the molecular orbital (mo) diagram for the be2 molecule. the bond order of be2 is calculated ncert problem 4.35 page no. 135 chemical bonding and molecular structure use molecular orbital theory to explain why the for the molecule be2: a) draw the molecular orbital diagram. Use MO diagrams and the bond order from them to answer ... Answer to: Use MO diagrams and the bond order from them to answer each of the following questions. (a) Is O2 stable or unstable? (b) Is Be2+...1 answer · Top answer: The MO diagram of O2O2 and Be+2Be2+ are drawn below. MO of Oxygen MO of Be2^+ a. The bond order of {eq} m... Molecular Orbital Diagram For Li2 - Wiring Diagrams Molecular orbital energy level of Li2. MO diagrams for Diatomic Molcules. Overview. In this section, we will compare MO diagrams for diatomic molecules X-X, from Li2 to Ne2. Molecular orbital diagram of Li2 & Be2: Number of electrons in Li2 molecule =6. Li2 = σ1s2,σ*1s2,σ2s2. Nb=4, Na=2. B.O = (Nb- Na). B.O = ().
Be2+ molecular orbital diagram. Stanford University UNK the , . of and in " a to was is ) ( for as on by he with 's that at from his it an were are which this also be has or : had first one their its new after but who not they have – ; her she ' two been other when there all % during into school time may years more most only over city some world would where later up such used many can state about national out known university united … Be2 Molecular Orbital Diagram from the above mo diagram we can see that number of elctrons in the bonding and antibonding orbital is same and hence be does not form be2 molecule (for.may 04, · turning to [be2]^- we have [be2]^- 5 valence e⁻s: σ1 (2e⁻) σ2 (2e⁻) π1 (1e⁻) σ3 (0e⁻) π2* (0e⁻) σ4* (e⁻) bond order = ½ [σ (bonding e⁻) - σ (antibonding e⁻)] so if we take the bonding … (Solved) - molecular orbital diagram. li2 is stable but ... 1. Extra Credit: Use molecular orbital theory and bond order to explain why the Be2 molecule does not exist. To be considered for credit, both the MO diagram and explanation must be completed. The molecule Be2 does not actually exist because... Molecular Orbital Diagram Ne2 - Diagram Niche Ideas Molecular orbital diagram ne2.If ne 2 did form, it would be diamagnetic. Once you have the molecular orbitals and their energy ordering the ground state configuration is found by applying the pauli principle, the what is the molecular orbital diagram for the diatomic neon molecule, ne2?
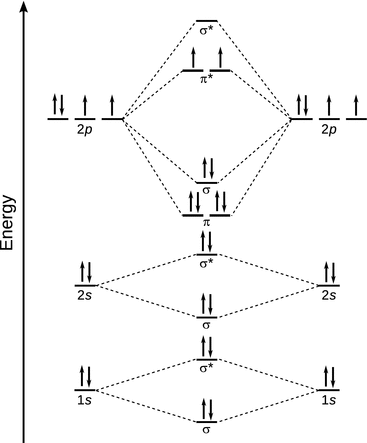
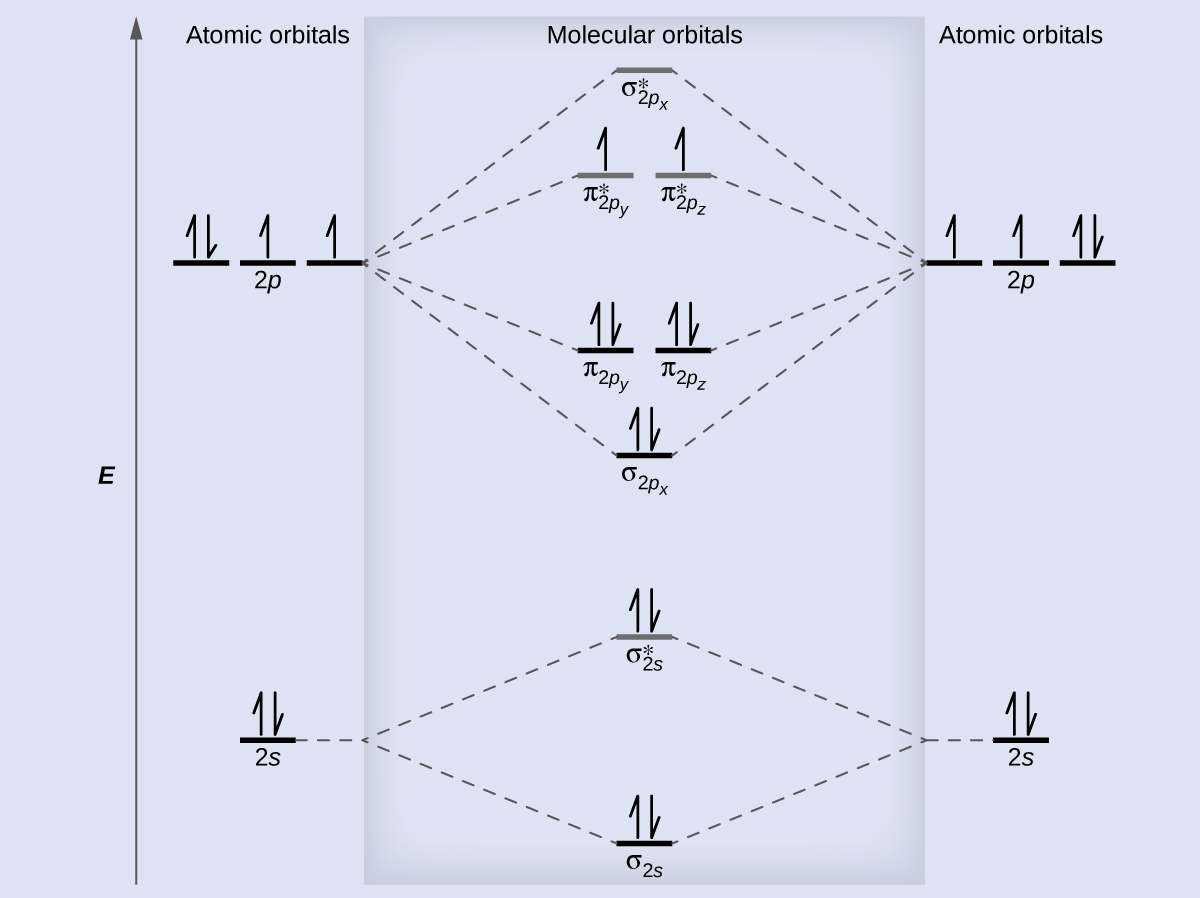
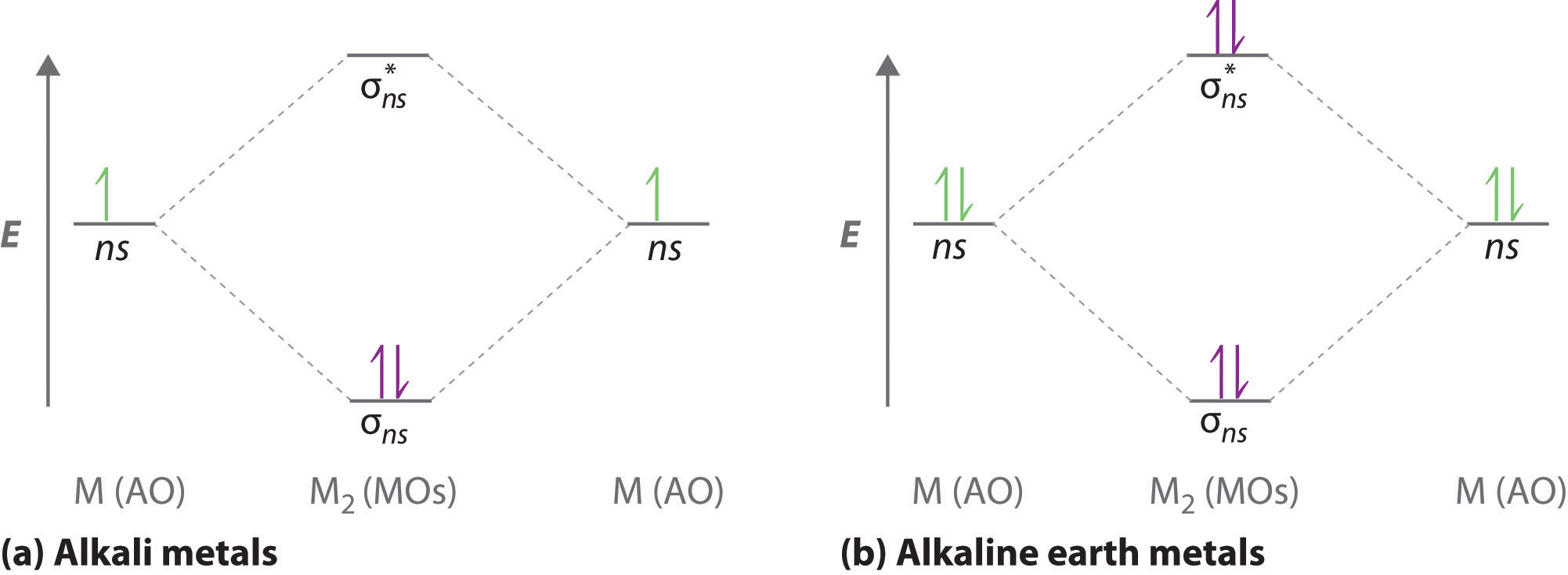
What is molecular orbital theory discuss LCAO method ... What are bonding and antibonding molecular orbitals describe LCAO method for their formation? The MO σ formed by the addition of atomic orbitals is called the bonding molecular orbital while the molecular orbital σ' formed by the subtraction of atomic orbital is known as anti bonding molecular orbitals. Why is be2 not formed? Bond order of Be2 is A 1 B 2 C 3 D 0 class 11 chemistry CBSE Bond order is equal to half of the difference between the number of electrons in bonding (. N b. ) and antibonding molecular orbitals (. N a. ). Complete Solution : B e 2. molecule will be formed by the overlapping of atomic orbitals of two beryllium atoms. A Be atom has four electrons. Be2 Molecular Orbital Diagram - schematron.org Answer to Draw an MO energy diagram and predict the bond order of Be2+ and Be2−. Do you expect these molecules to exist in the. Even rather simple molecular orbital (MO) theory can be used to predict which we start reading from the bottom of the diagram because this is how MO diagrams are constructed, Diberyllium, Be2, has a bond order of zero and is unknown. 7.7 Molecular Orbital Theory - Chemistry Fundamentals The relative energy levels of atomic and molecular orbitals are typically shown in a molecular orbital diagram (Figure 7.7.9). For a diatomic molecule, the atomic orbitals of one atom are shown on the left, and those of the other atom are shown on the right. Each horizontal line represents one orbital that can hold two electrons.
PDF MO Diagrams for Diatomic Molecules Summary MO Theory • LCAO-MO Theory is a simple method for predicting the approximate electronic structure of molecules. • Atomic orbitals must have the proper symmetry and energy to interact and form molecular orbitals. • Photoelectron spectroscopy provides useful information on the energies of atomic orbitals. • Next we'll see that symmetry will help us treat larger molecules in What Is The Bond Order Of B2 1So the bond order of B2 is equal to 1, which you can get by drawing the molecular orbital diagram and performing the equation Bond Order = . 5 * (# of bonding electrons - # of antibonding electrons). However, when you draw the Lewis structure of B2, you get a triple bond.Nov 11, 2016 Molecular Orbital Diagram Be2 - schematron.org + and Be2.A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular. 1. Solved 1. Draw the molecular orbital energy level diagram Draw the molecular orbital energy level diagram for each of the following species Be2+, Be2, and Be2-. Indicate theirnumbers of unpaired electron and mention ...1 answer · Top answer: Atomic number of Be is 4 so it has 4 electrons . Electronconfiguration of Be is 1s2 ,2s2 Bondorder in Be2 = (1/2) [ bondedelectrons - anti bonding electrons ...
Molecular Orbital (MO) Diagram of Be2 - YouTube Molecular Orbital Diagram for Beryllium Dimer (Be2)Fill from the bottom up, with 4 electrons total.Bonding Order is 0, meaning it does not bond, and it is di...
Using the molecular orbital theory, why does a Be2 ... Answer (1 of 8): Well, as the first point it is to be noted that an atom forms a molecule in order to get stabilised. In other word we can say that in order to form a molecule the energies of the atomic orbitals should be lowered in the molecule. Now, we know that in a molecule the atomic orbita...
(PDF) Chemistry - Gilbert | Tín Phạm - Academia.edu Academia.edu is a platform for academics to share research papers.
What is the molecular orbital diagram for B_2? | Socratic Before we can draw a molecular orbital diagram for B₂, we must find the in-phase and out-of-phase overlap combinations for boron's atomic orbitals. Then we rank them in order of increasing energy. We can ignore the 1s orbitals, because they do not contain the valence electrons. Each boron atom has one 2s and three 2p valence orbitals. The 2s orbitals will overlap to form 2sσ and 2sσ ...
SOLVED:Draw an MO energy diagram and predict the bond ... Problem 43 Hard Difficulty. Draw an MO energy diagram and predict the bond order of Be2+ and Be2-. Do you expect these molecules to exist in the gas phase?12 Jul 2021
CHAPTER 5: MOLECULAR ORBITALS 5.7 a. The energy level diagram for NO is on the right. The odd electron is in a π2p* orbital. b ...29 pagesMissing: be2+ | Must include: be2+
Molecular Orbital Diagram Be2 Molecular Orbital Diagram Be2 Even rather simple molecular orbital (MO) theory can be used to predict which we start reading from the bottom of the diagram because this is how MO diagrams are constructed, Diberyllium, Be2, has a bond order of zero and is unknown.
Draw the molecular orbital diagram for: (i) Be2 (ii) O2 ... Draw the molecular orbital diagram for: (i) Be2 (ii) O2 and predict bond order, stability and magnetic properties.
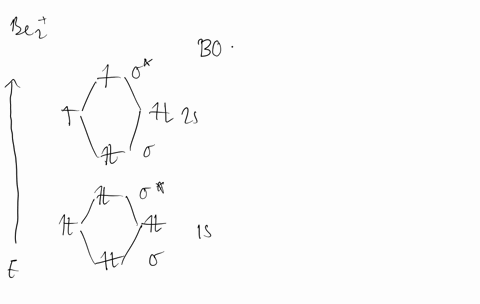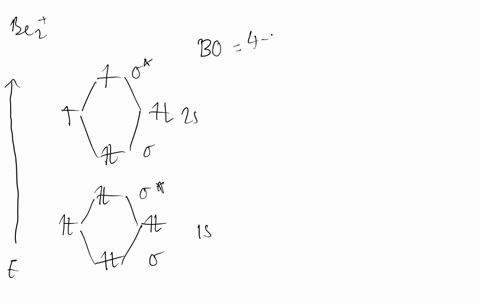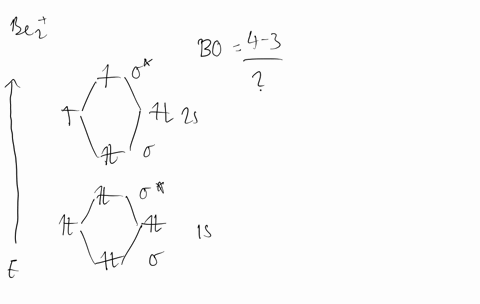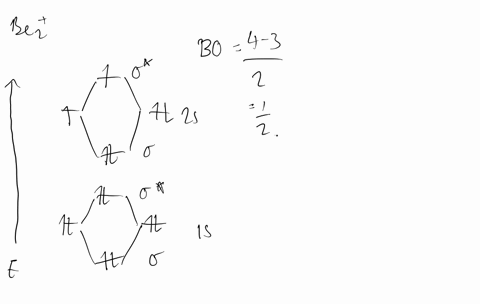
Molecular Orbital Theory - Build Be2+ - YouTube For the ion Be2+:a) Draw the molecular orbital diagram.b) Calculate the bond order.c) Would this ion exist?d) Write the electron configuration of the ion————...
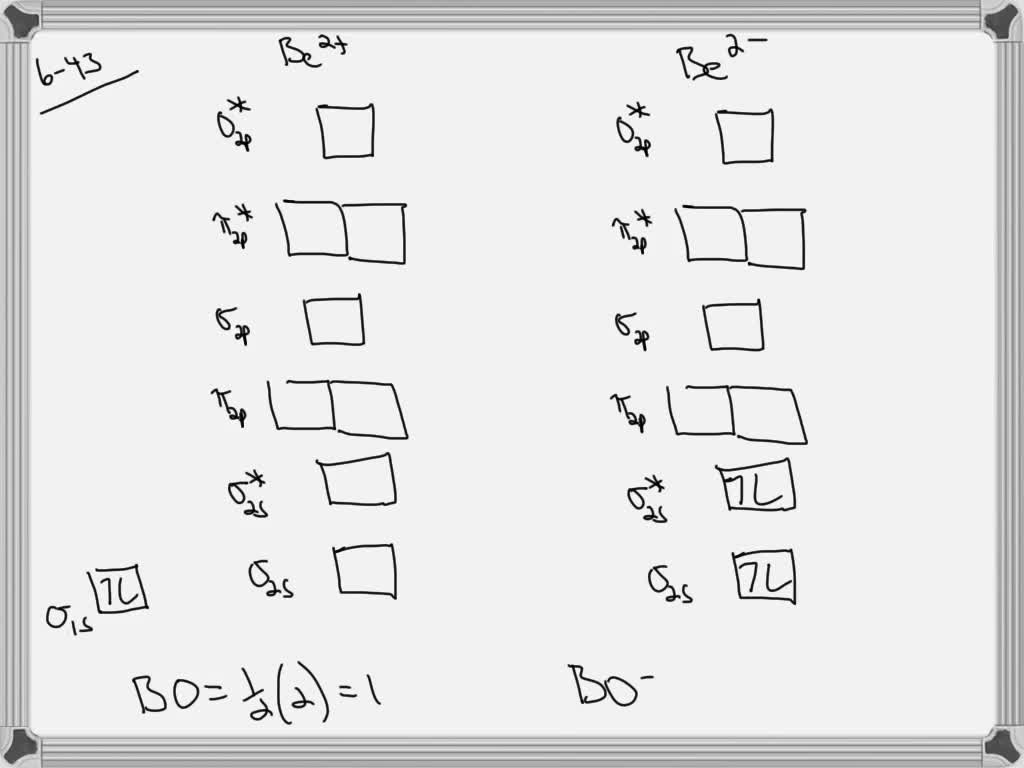
draw an mo energy diagram and predict the bond order of be2 and be2 do you expect these molecules to
Draw the molecular orbital (MO) electron d... | Clutch Prep Construct the molecular orbital diagram for Be2- while applying the necessary rules in filling up the orbitals. Step 1: Calculate the total number of valence electrons present. Step 2: Draw the molecular orbital diagram. Recall that the bonding MOs are those without an asterisk (e.g., σ1s), while the antibonding MOs are those with an asterisk ...
34 No Molecular Orbital Diagram Wiring Diagram Database ... Molecular orbital theory is also able to explain the presence of figure \(\ pageindex{6}\): molecular orbital energy level diagram for hcl. to describe the bonding in the cyanide ion (cn −). mix atomic orbitals on different atoms to get molecular orbitals. the resul7ng mo diagram looks like this. cn- (cyanide ion), no (nitrosonium ion). 29 ...
Why does the molecular orbital diagram for Be2+ consist of ... For completeMO diagrams, you use the total number of electrons. The 1s electrons of O2, N2, etc. are used to fill up the sigma(1s) and sigma(1s)* molecular orbitals. Similarly, with Be2+as well, there are 2(4) - 1 = 7 total electrons if you're filling out a complete MO diagram. 12 Share ReportSave level 2 Op· 3y
Solution Manual - Chemistry-4th Ed. (mcmurry) [vylyo7yvgvnm] It is possible for a molecular compound to be a strong electrolyte. For example, HCl is a molecular compound when pure but dissociates completely to give H+ and Cl- ions when it dissolves in water. 4.38 (a) K2CO3 contains 3 ions (2 K+ and 1 CO32-). The molar concentration of ions = 3 x 0.750 M = 2.25 M. (b) AlCl3 contains 4 ions (1 Al3+ and 3 Cl-).
45 b2 2- molecular orbital diagram - Modern Wiring Diagram Using the molecular orbital theory, why does a Be2 molecule not exist? The orbital diagram for a diatomic molecule is. To find the bond order, add the 15 electrons in the molecular orbitals (the blue-colored energy levels According to molecular orbital theory,molecular orbital diagram for helium molecule can be given as.
Use molecular orbital theory to explain why the Be2 ... Use molecular orbital theory to explain why the Be2 molecule does not exist. Answer. The electronic configuration of Beryllium is 1s 2 2s 2. From the electronic configuration it is clear that there is no singly filled atomic orbital present in beryllium.
Solved Construct the molecular orbital diagram for Be2 ... Solved Construct the molecular orbital diagram for Be2. Note | Chegg.com. Construct the molecular orbital diagram for Be2. Note that the 1s orbitals are not shown. Be Ho Be Answer Bank IL | Identify the bond order. O 0 O os O 1s. Question: Construct the molecular orbital diagram for Be2. Note that the 1s orbitals are not shown.
Molecular Orbital Diagram For Li2 - Wiring Diagrams Molecular orbital energy level of Li2. MO diagrams for Diatomic Molcules. Overview. In this section, we will compare MO diagrams for diatomic molecules X-X, from Li2 to Ne2. Molecular orbital diagram of Li2 & Be2: Number of electrons in Li2 molecule =6. Li2 = σ1s2,σ*1s2,σ2s2. Nb=4, Na=2. B.O = (Nb- Na). B.O = ().
Use MO diagrams and the bond order from them to answer ... Answer to: Use MO diagrams and the bond order from them to answer each of the following questions. (a) Is O2 stable or unstable? (b) Is Be2+...1 answer · Top answer: The MO diagram of O2O2 and Be+2Be2+ are drawn below. MO of Oxygen MO of Be2^+ a. The bond order of {eq} m...
34 Be2 Mo Diagram Wiring Diagram Database - Dubai Burj ... How To Make The Molecular Orbital Diagram For Be2: Does The Molecule Exist? this video discusses how to draw the molecular orbital (mo) diagram for the be2 molecule. the bond order of be2 is calculated ncert problem 4.35 page no. 135 chemical bonding and molecular structure use molecular orbital theory to explain why the for the molecule be2: a) draw the molecular orbital diagram.











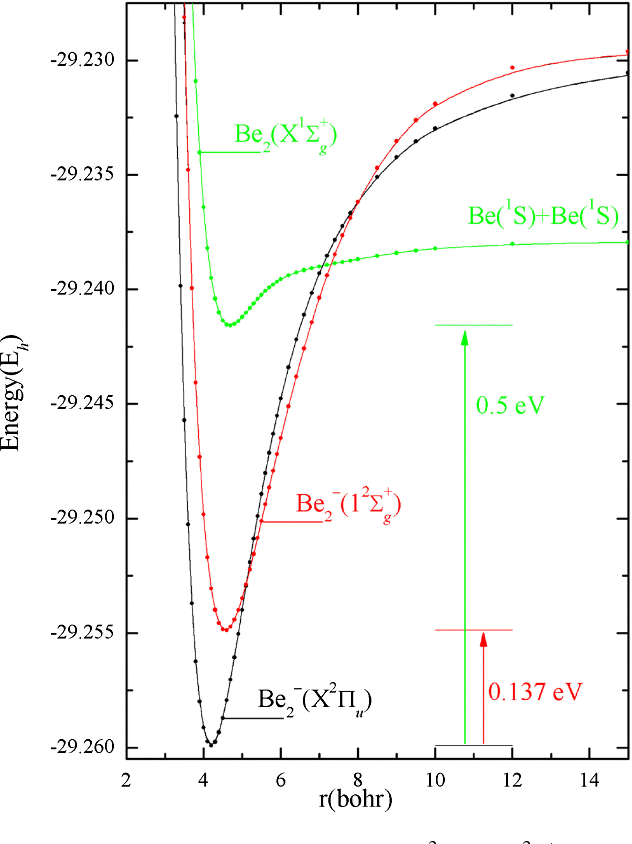


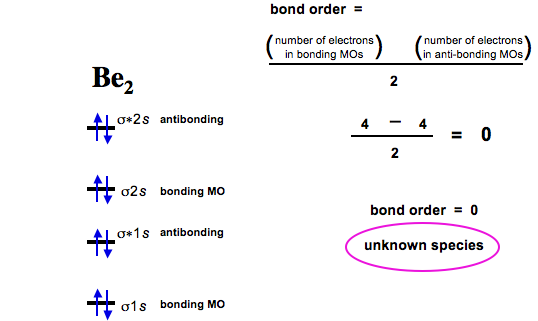
0 Response to "39 be2+ molecular orbital diagram"
Post a Comment