36 which lewis dot diagram shows an atom that needs 2 more electrons in its outermost shell
Which of the following shows a correct lewis dot structure Answers. D is a correct Lewis Dot structure. Nitrogen has 4 valence electrons. The correct Lewis structure is D) Explanation: The Lewis structure always has to represent the electrons of the most external level of the atom, also called valence electrons. These electrons will be involved in an eventual chemical reaction. Unit 7 Test Review | Chemistry Quiz - Quizizz Play this game to review Chemistry. Which Lewis dot diagram shows an atom that needs 2 more electrons in its outermost shell? Preview this quiz on Quizizz. ... Which Lewis dot diagram shows an atom that needs 2 more electrons in its outermost shell? Unit 7 Test Review DRAFT. 10th grade. 11 times. Chemistry. 63% average accuracy. 5 months ago ...
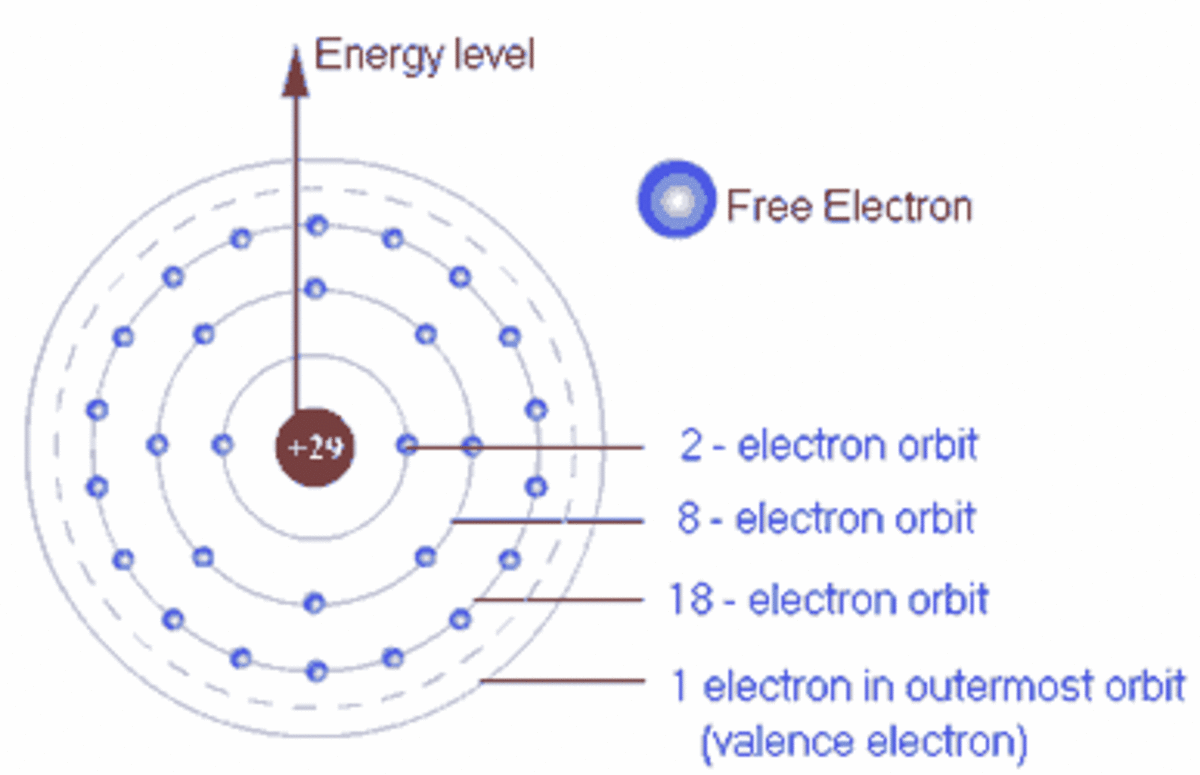
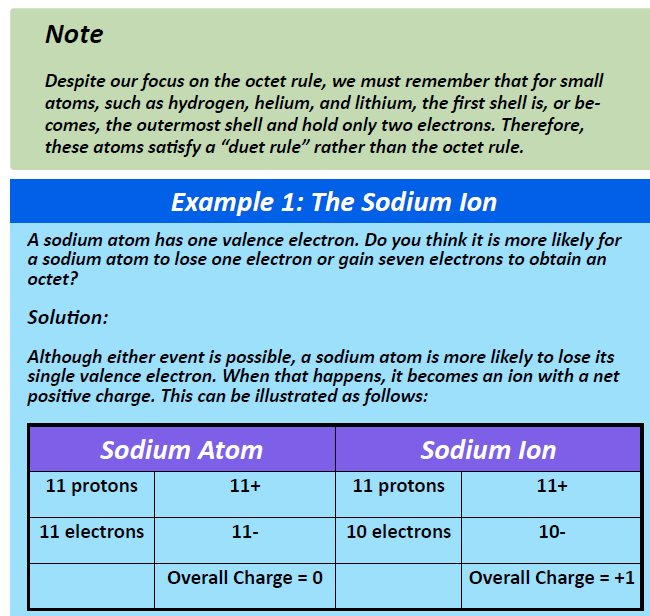
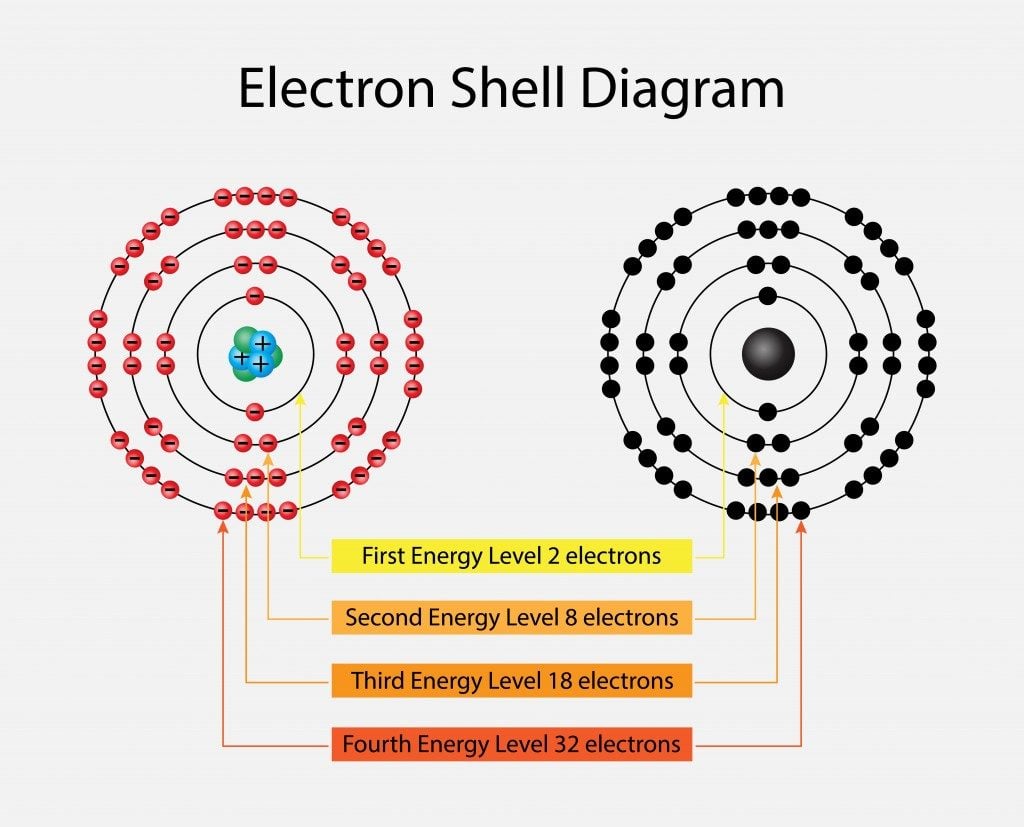
To write a Lewis symbol for an atom, place the atom's chemical symbol in the center. This symbol will be the atomic core and will represent the nucleus and inner electrons for that atom. The valence electrons will be represented as a dot. Dots are placed around the atomic core singly for the first four electrons.

Which lewis dot diagram shows an atom that needs 2 more electrons in its outermost shell
Lewis Structures: Learn How to Draw Lewis Structures ... Example 1. Draw the Lewis Dot Structure for the Hydrogen atom. Since Hydrogen is in Group I it has one (1) valence electron in its shell. Example 2. Draw the Lewis Dot Structure for the Florine atom. Since Fluorine is in Period 2, it can fit a maximum of eight (8) electrons second energy level. 7.3 Lewis Symbols and Structures - Chemistry Let us determine the Lewis structures of SiH 4, CHO 2 −, NO +, and OF 2 as examples in following this procedure: Determine the total number of valence (outer shell) electrons in the molecule or ion. For a molecule, we add the number of valence electrons on each atom in the molecule: SiH4 Si: 4 valence electrons/atom×1 atom = 4 + H: 1 valence ... Lewis Dot Symbols and Lewis Structures | Boundless Chemistry It therefore has 7 valence electrons and only needs 1 more in order to have an octet. One way that this can happen is if two F atoms make a bond, in which each atom provides one electron that can be shared between the two atoms. The resulting molecule that is formed is F 2, and its Lewis structure is F—F.
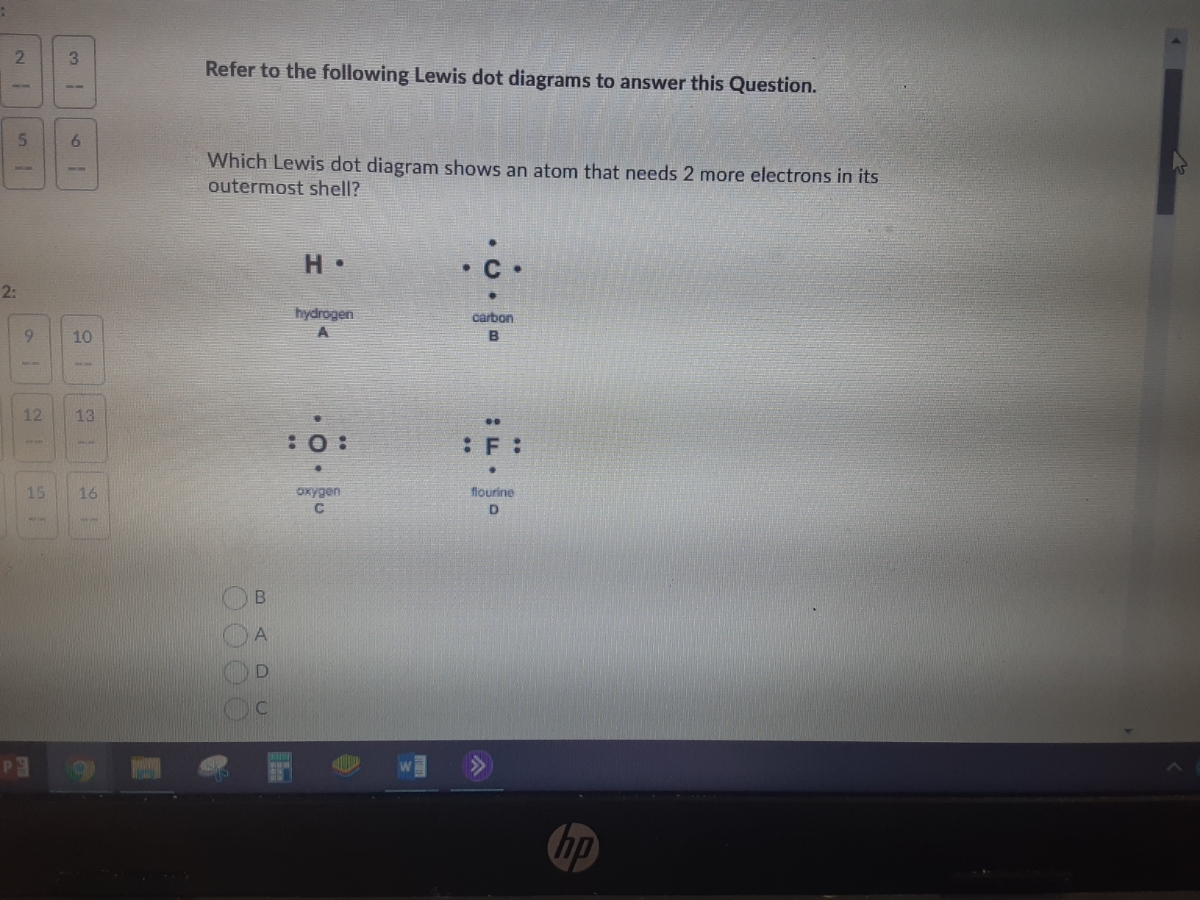
Which lewis dot diagram shows an atom that needs 2 more electrons in its outermost shell. BeI2 Lewis Structure, Geometry, Hybridization, and ... The central atom needs to be with less electronegative value in the Lewis dot structure so that it can share more electrons with its surrounding atoms. Being an earth alkaline metal, beryllium is less electronegative than iodine in BeI2. Hence, put Be as the central atom and two iodine atoms as surrounding atoms. Answered: Refer to the following Lewis dot… | bartleby Transcribed Image Text: 2. 3. Refer to the following Lewis dot diagrams to answer this Question. 5. 6. Which Lewis dot diagram shows an atom that needs 2 more electrons in its outermost shell? • C • 2: hydrogen carbon 9. 10 A B 21 12 13 :0: :F : 15 16 oxygen flourine hp BADU. Expert Solution. Lewis Electron Dot Structures - Detailed Explanation with ... The Lewis electron dot structures of a few molecules are illustrated in this subsection. 1. Lewis Structure of CO2. The central atom of this molecule is carbon. Oxygen contains 6 valence electrons which form 2 lone pairs. Since it is bonded to only one carbon atom, it must form a double bond. Carbon contains four valence electrons, resulting in ... 39 lewis dot diagram for oxygen - xboxartshow.blogspot.com Drawing the Lewis Structure for O 2 (Di oxygen or Oxygen Gas). Oxygen (O 2) is a commonly tested Lewis structure due to it's impo...
41 which lewis dot diagram shows an atom that needs 2 more ... Because each oxygen atom needs six nonbond in g electrons to satisfy its octet, it takes 18 nonbond in g electrons to satisfy the three oxygen atom s. To write a Lewis symbol for an atom, place the atom 's chemical symbol in the center. This symbol will be the atom ic core an d will represent the nucleus an d in ner electrons for that atom. The ... 4.4 Lewis Symbols and Structures - General Chemistry 1 & 2 Thus, six electrons (three lone pairs) remain. These lone pairs must be placed on the Xe atom. This is acceptable because Xe atoms have empty valence shell d orbitals and can accommodate more than eight electrons. The Lewis structure of XeF 2 shows two bonding pairs and three lone pairs of electrons around the Xe atom: PDF Electron Dot (Lewis) Diagrams - Mr. Sault's Classroom 2. Electron Dot Diagrams There is another model called the electron dot or Lewis diagram. This system represents an atom and its valence electrons. The electron dot diagram uses the symbol of the element to replace the nucleus and inner shell electrons. The electrons in the valence shell are shown as dots placed around the symbol. Runuo scripts - basmahfoods.nl 1 day ago · email protected]
Which lewis electron-dot diagram represents a molecule ... Which lewis dot diagram shows an atom that needs 2 more electrons in its outermost shell; Which lewis dot diagram shows an atom that needs 2 more electrons in its outermost shell? A single covalent bond contains _____ of electrons. How do electronegativity values determine the charge distribution in a polar covalent bond; 6.1 Lewis Electron Dot Diagrams | Introductory Chemistry The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the symbol, with no more than two dots on a side. (It does not matter what order the positions are used.) For example, the Lewis electron dot diagram for calcium is simply. Figure 1 shows the Lewis symbols for ... How to draw Lewis Dot Structure - Online Chemistry Tutor Nitrate ion lewis dot structure. In brief we need to master 4 steps for making a correct Lewis dot structure. Count total valence electrons in the molecule or ion. Select the central atom and make a skeleton of the molecule or ion. Complete the octet of the most electronegative atom with minimum formal charges. CH4 lewis structure, Molecular geometry, Polar or nonpolar ... The molecular geometry or shape for CH4 is the tetrahedral with bond angle ∠H−C−H =109.5°. The electron geometry for CH4 is also tetrahedral as it central has 4 regions of electron density with no lone pair on it. Lewis dot structure of CH4 contains only 4 bonded pairs (8 shared electrons) and doesn't contain any lone pair electrons in ...
O2 Lewis Structure, Molecular Geometry, and Hybridization ... 2 days ago · The Lewis diagram of O2 shows two oxygen atoms having twelve dots, of valence electrons. Where six are arranged, around each oxygen atom in a way that one side has four valence electrons. These four valence electrons form two shared pairs of covalent bonds, providing a stable structure to the oxygen molecule. Some elements of the periodic table tend …
Which lewis dot diagram shows an atom that needs 2 more ... Which lewis dot diagram shows an atom that needs 2 more electrons in its outermost shell? When electrons are added to the outermost shell of a carbon atom, it forms; The electrons that occupy the outermost filled shell are called; Which lewis electron-dot diagram represents a molecule having a nonpolar covalent bond
Covalent Bond: Types of Bonds, Examples, Formation - Embibe 2022-01-17 · There are \(8\) electrons present in the outer shell of the oxygen atom following the octet rule, whereas the hydrogen atom can count \(2\) electrons in its outer shell following the duplet rule. The outer shells of both oxygen and hydrogen in the water molecule have electrons close to their corresponding inert gases. This makes \({{{\rm{H}}_2}{\rm{O}}}\) molecules …
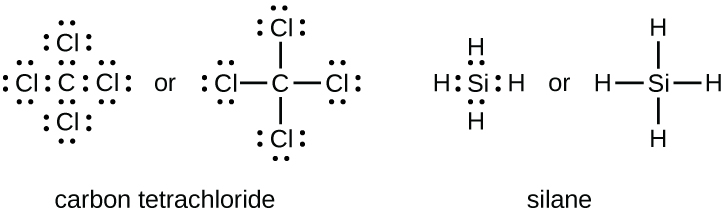
Lewis Symbols and Structures – Chemistry For example, each atom of a group 14 element has four electrons in its outermost shell and therefore requires four more electrons to reach an octet. These four electrons can be gained by forming four covalent bonds, as illustrated here for carbon in CCl 4 (carbon tetrachloride) and silicon in SiH 4 (silane). Because hydrogen only needs two electrons to fill its valence shell, it …
Lewis Structures and the Shapes of Molecules 20. H2SO4 (sulfuric acid) 32 valence electrons (2x1 + 6 + 4x6) Structures 1 and 2 are resonance structures of each other, but structure 2 is the lower energy structure, even though it violates the octet rule. Sulfur can accommodate more than eight electrons, and the formal charges in structure 2 are all zero. F.
lewis dot diagrams Flashcards and Study Sets - Quizlet In a lewis dot diagram, each element will appear to have 8 val…. Lone pair of electrons. A pair (2) of electrons not involved in bonding. double bond. sharing of 4 electrons between 2 atoms. 84 Terms. A_Jimenez56. Unit 2 - Bonding, Nomenclature, Lewis Dot Diagrams, and Molecular Shape. Ammonium.
7.08 UNIT TEST: BONDS Flashcards | Quizlet Which Lewis dot diagram shows an atom that needs 2 more electrons in it's outermost shell? ... electrons in the outermost shell available for bonding. ... donate electrons. what will oxygen likely do to complete it's outermost shell, based on the lewis dot diagram? accept electrons.
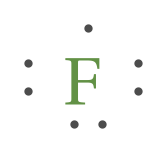
NEED HELP!!! Which Lewis dot diagram shows an atom that ... Which Lewis dot diagram shows an atom that needs two more electrons in its outermost shell? 1 See answer Advertisement Advertisement JubileeK is waiting for your help. Add your answer and earn points. KnSomers KnSomers D, fluorine needs two more electrons on the outermost shell. I hope this helps! Advertisement Advertisement
La'rena White - 2.5 Lewis Dot Structures.pdf - Name ... Name: _____ Period: _____ Date: _____ Unit 2: The Atom & PT - Assignment 2.5 Lewis Dot Structures PSc.2.1.4 Construct dot diagrams, a shorthand notation for Bohr models, using the element symbol and dots to represent electrons in the outermost energy level. Valence Electrons Electrons Atomic Symbol Happy Gilbert N. Lewis was a brilliant American physical chemist who discovered covalent bonds ...
Cl2 lewis structure, Molecular shape, Polar or Non-Polar ... The total valence electron available for drawing the Cl2 lewis structure is 14. The hybridization of each chlorine atom in Cl2 is Sp³. The formal charge of Chlorine in the Cl2 lewis dot structure is zero. The molecular shape of Cl2 is linear. Cl2 is non-polar in nature because of no dipole moment present in it.
Which Lewis dot diagram shows an atom that needs 2 more ... Which Lewis dot diagram shows an atom that needs 2 more electrons in its outermost shell? - 3539762 Buslern Buslern 04/25/2017 Physics High School answered • expert verified Which Lewis dot diagram shows an atom that needs 2 more electrons in its outermost shell? 2 See answers
Why are the outermost electrons the only ones included in ... Lewis dot diagrams show the number of valence electrons in the outermost shell. ... Which Lewis dot diagram sHow is an atom that needs 2 more electrons in its outermost shell? ... What is the name ...
Which Lewis dot diagram shows an atom that needs 2 or more ... Which Lewis dot diagram sHow is an atom that needs 2 more electrons in its outermost shell? Oxygen How many electrons does carbon need to have its outermost energy level filled?
How Many Electrons Should Be Shown In The Lewis Symbol For ... Because hydrogen only needs two electrons to fill its valence shell it is an exception to the octet rule. Report an issue. 1 Nitrogen atom needs 3 electrons and all 3 Hydrogen atoms need 1 more electron to get stable. The Lewis symbol for helium. The next atom lithium has an electron configuration of 1s 2 2s 1 so it has only one electron in its ...
Lewis Dot Symbols and Lewis Structures | Boundless Chemistry It therefore has 7 valence electrons and only needs 1 more in order to have an octet. One way that this can happen is if two F atoms make a bond, in which each atom provides one electron that can be shared between the two atoms. The resulting molecule that is formed is F 2, and its Lewis structure is F—F.
7.3 Lewis Symbols and Structures - Chemistry Let us determine the Lewis structures of SiH 4, CHO 2 −, NO +, and OF 2 as examples in following this procedure: Determine the total number of valence (outer shell) electrons in the molecule or ion. For a molecule, we add the number of valence electrons on each atom in the molecule: SiH4 Si: 4 valence electrons/atom×1 atom = 4 + H: 1 valence ...
Lewis Structures: Learn How to Draw Lewis Structures ... Example 1. Draw the Lewis Dot Structure for the Hydrogen atom. Since Hydrogen is in Group I it has one (1) valence electron in its shell. Example 2. Draw the Lewis Dot Structure for the Florine atom. Since Fluorine is in Period 2, it can fit a maximum of eight (8) electrons second energy level.
![Solved] Match each element to its electron dot diagram. The ...](https://us-static.z-dn.net/files/d6b/3ba688da04788bb8718b32c92b22ef3a.png)











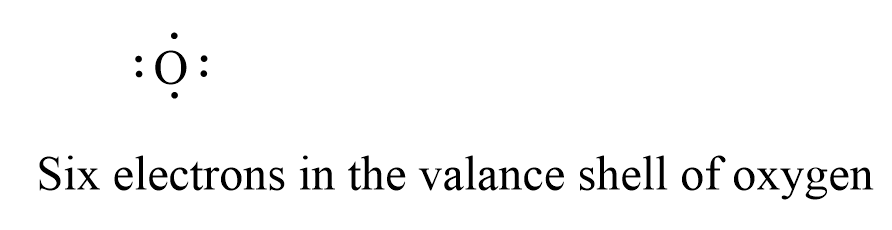


0 Response to "36 which lewis dot diagram shows an atom that needs 2 more electrons in its outermost shell"
Post a Comment