36 orbital diagram of titanium
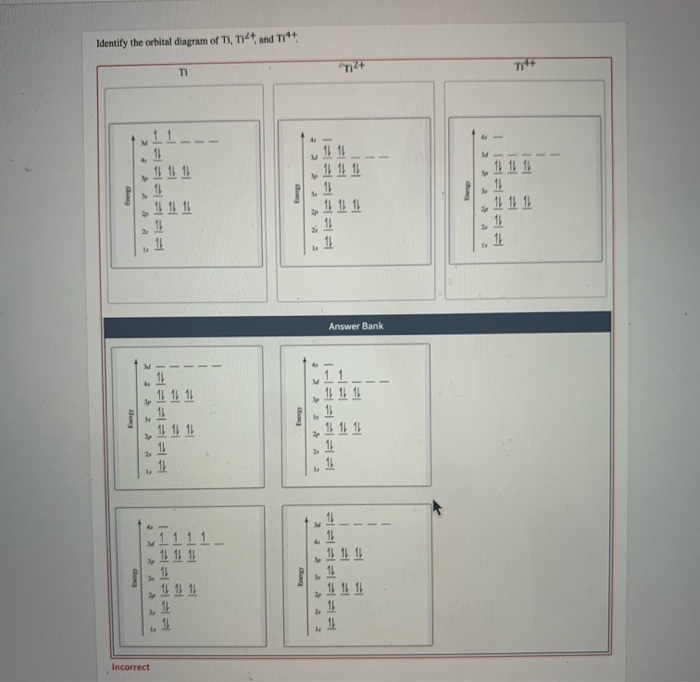
Titanium (Ti) - Periodic Table (Element Information & More) Yes, Titanium is a transition metal because it has incompletely filled d-orbital in its ground state.. Let me explain the exact meaning of this. According to the definition of transition metals; The element should compulsorily have incomplete d-orbitals, either in their ground state (M) or most common oxidation states (M 1+, M 2+, etc) then only they are called transition metals. Orbital Diagram For Ti2+ The probability of finding an electron at the nucleus is 0. However, once the 4s orbital is filled, it becomes higher in energy than the 3d orbitals. This means that when titanium loses electrons, it does so. Answer to Construct the orbital diagram of each atom or ion. Ti Ti2+ Ti4+.
OneClass: What is the orbital diagram of each atom or ion ... What is the orbital diagram of each atom or ion? Ti, Ti 2+, Ti 4+ Answer +20. Watch. 1. answer. 0. watching. 755. views. For unlimited access to Homework Help, a Homework+ subscription is required. Christian Garcia Lv10. 26 Jan 2021. Unlock all answers. Get 1 free homework help answer. Unlock. Already have an account? ...

Orbital diagram of titanium
Alveolar process - Wikipedia Terminology. The term alveolar ('hollow') refers to the cavities of the tooth sockets, known as dental alveoli. The alveolar process is also called the alveolar bone or alveolar ridge. The curved portion is referred to as the alveolar arch. The alveolar bone proper, also called bundle bone, directly surrounds the teeth. The term alveolar crest describes the extreme rim of the bone … HCOOH Lewis Structure, Molecular Geometry, Hybridization ... 25.2.2022 · The orbital diagram of formic acid, which represents the sigma bonds, is shown below. We can observe from the orbital diagram is that the carbon atom is the sp2 hybridized and one of the oxygen atoms is also sp2 hybridized whereas another oxygen atom bonded to hydrogen and carbon atom, is sp3 hybridized. Ti3 electron configuration | electron configuration for ti ... Orbital Diagram of Titanium (Ti), electron configuration . Electronic configuration of Mn 2+ is [Ar] 18 3d 5. Electronic configuration of Fe 2+ is [Ar] 18 3d 6 . It is known that half-filled and fully-filled orbitals are more stable ; Electron Configuration: [Xe]4f145d96s1 Oxidation State: 2,4 Crystal Structure: cubic. Discovered by J. Scaliger ...
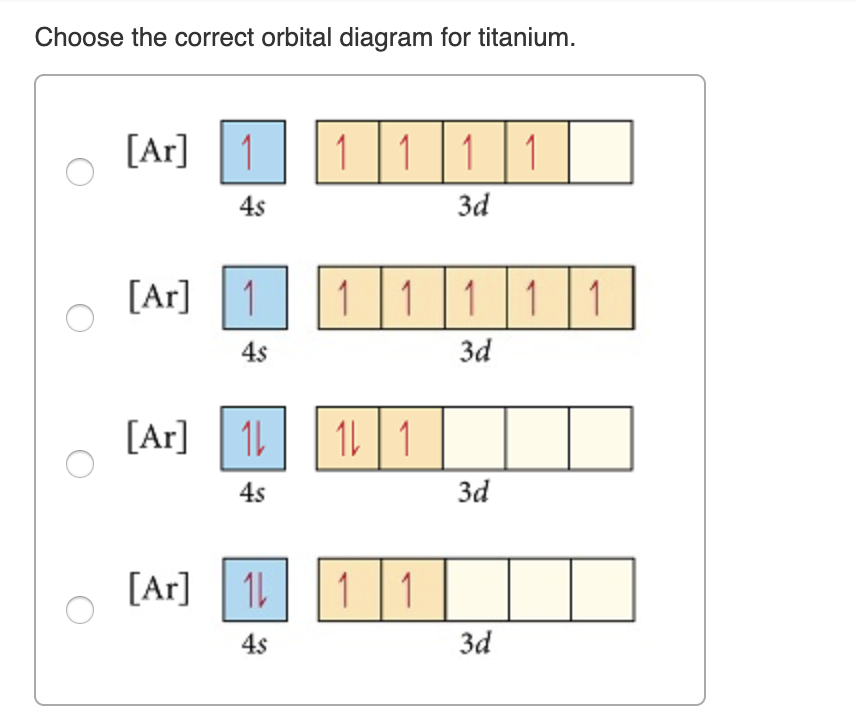
Orbital diagram of titanium. Exam 4 Review: Ch.8-9 Flashcards | Quizlet Exam 4 Review: Ch.8-9. Electromagnetic radiation with a wavelength of 745 nm appears as red light to the human eye. The energy of one photon of this light is ________ J. Calculate the wavelength (in nm) of the blue light emitted by a mercury lamp with a frequency of 6.19 × 10^14 Hz. Nice work! Module Two Chem 101 Problems Flashcards | Quizlet Start studying Module Two Chem 101 Problems. Learn vocabulary, terms, and more with flashcards, games, and other study tools. chem 111-exam 2 Flashcards - Quizlet Choose the orbital diagram that represents the ground state of N. orbital diagram where 1s and 2s orbitals contain 1 pair of electrons each. 2p orbitals are empty. orbital diagram where 1 s and 2 s orbitals contain 1 pair of electrons each. 2 p orbitals contain 3 pairs of electrons. Orbital Diagram of Titanium (Ti), electron configuration ... This video shows how to draw the orbital diagram of Titanium (Ti). It also shows how to write the electron configuration of titanium and the shorthand noble...
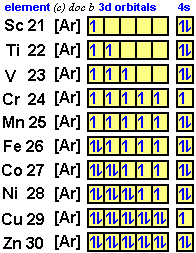
Orbital Diagram & Electron Configuration for uranium - YouTube Emporia High School Muldoon Short Question -t2 - Chemhoper Titanium exists as several isotopes. The mass spectrum of a sample of titanium gave the data : Calculate the relative atomic mass of titanium to two decimal places. Ans ... Sketch the orbital diagram of the valence shell of a bromine atom (ground state) on the energy axis provided. PDF Electron Configurations and Orbital Diagrams key 1. Describe the two differences between a 2p x orbital and a 3p y orbital. The 2px orbital lies on the x-axis. The 3py orbital lies on the y-axis and is larger than the 2px orbital. 2. The lobes of a p orbital disappear at the nucleus. What does this tell us about electrons in p orbitals? Scandium(Sc) electron configuration and orbital diagram Orbital diagram for scandium(Sc) Scandium(Sc) excited state electron configuration. Atoms can jump from one orbital to another orbital by excited state. This is called quantum jump. Ground state electron configuration of scandium(Sc) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 1 4s 2. The valency of the element is determined by electron configuration in the ...
Chem4Kids.com: Titanium: Orbital and Bonding Info Titanium atomic orbital and chemical bonding information. There are also tutorials on the first thirty-six elements of the periodic table. Check out the blackboard. That box on the left has all of the information you need to know about one element. It tells you the mass of one atom, how many ... PDF Electronic structure of titanium oxide clusters: TiO 51-3 ... highest occupied molecular orbital/lowest unoccupied mo-lecular orbital ~HOMO-LUMO! gap. The n1 vibrational fre-quency of the TiO2 ground state is measured to be 960 ~40! cm21. The EA of TiO 2 ~1.59 eV! is only slightly higher than that of TiO ~1.30 eV! while TiO3 has a very high EA of about 4.2 eV. The larger (TiO2)n clusters are all found to be Electron Affinity Chart (Labeled Periodic table + List) 26.4.2021 · Electron affinity of Titanium (Ti) 7.28: 23: Electron affinity of Vanadium (V) 50.91: 24: Electron affinity of Chromium (Cr) 65.21: 25: Electron affinity of Manganese (Mn) (-50) 26: Electron affinity of Iron (Fe) 14.78: 27: Electron affinity of Cobalt (Co) 63.89: 28: Electron affinity of Nickel (Ni) 111.65: 29: Electron affinity of Copper (Cu ... What is the electron configuration of "Ti"^(2+)? | Socratic This means that when titanium loses electrons, it does so from the 4s orbital first. Ti: 1s22s22p63s23p63d24s2 Therefore, the two electrons that are lost when the Ti2+ is formed will come from the 4s orbital, which means that the electron configuration of the cation is
Titanium Electron Configuration (Ti) with Orbital Diagram Jan 26, 2021 · Titanium Electron Configuration (Ti) with Orbital Diagram January 26, 2021 Leave a Comment Titanium Electron Configuration : Titanium is a chemical element that has a chemical symbol Ti.
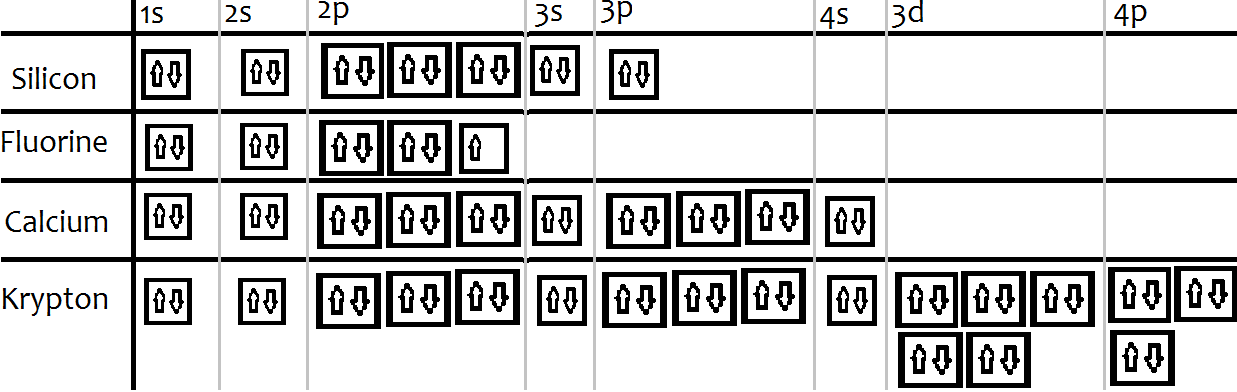
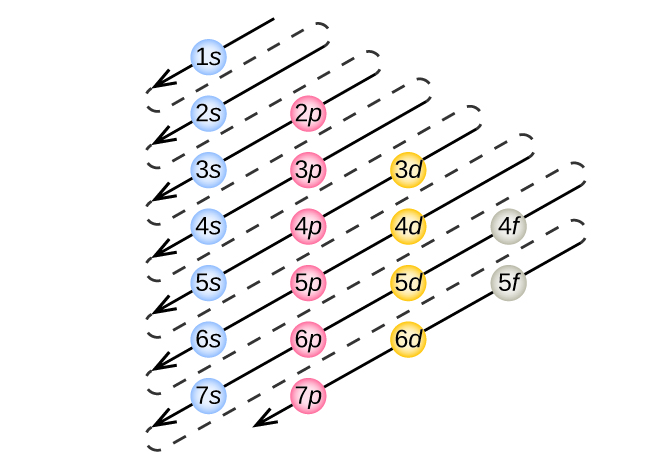
Orbital Diagrams Chemistry Tutorial - AUS-e-TUTE An orbital diagram, or orbital box diagram, is a way of representing the electron configuration of an atom. A box, line, or circle, is drawn to represent each orbital in the electron configuration. (using the Aufau Principle to order the orbitals and hence the boxes, lines or circles, as shown below) 1s. →. 2s.
Solved Construct the orbital diagram of each atom or ion ... Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. 100% (10 ratings) Transcribed image text: Construct the orbital diagram of each atom or ion. What is the electron configuration for titanium?
Clarifying Electron Configurations | Chemical Education ... Let's consider titanium (Z = 22). Its electron configuration is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 2, which the (n + l) rule correctly predicts. If the electron configuration depended solely on the orbital energies, we would expect: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 4 - with no electrons in the 4s orbital.
PDF Orbital diagram for ti - uploads.strikinglycdn.com orbital #4s, so you can see here However, once the #4s # orbital is filled, it becomes higher in energy than the orbital#3d #. This means that when titanium loses electrons, it does so from #4s # orbital first. #Ti: 1s^2 2s^2 2p^6 3s^2 3p^6 3d^2 4s^2# Therefore, the two electrons that get lost when
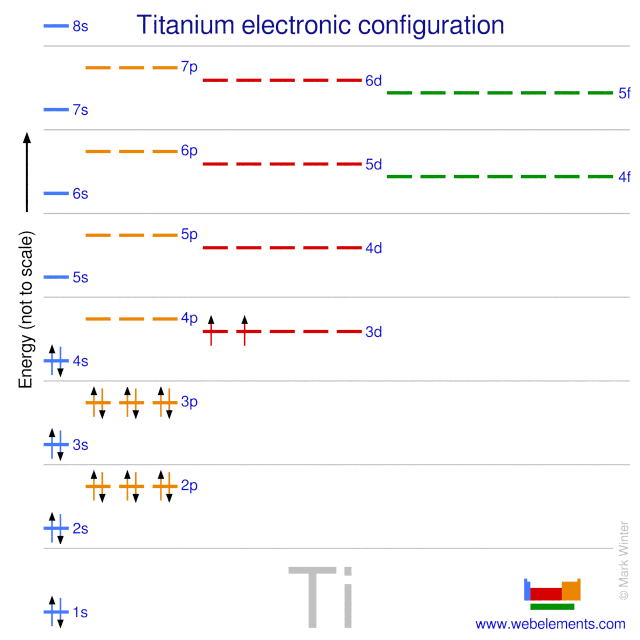
Electron configuration for Titanium (element 22). Orbital ... Titanium electron configuration. Ti (Titanium) is an element with position number 22 in the periodic table. Located in the IV period. Melting point: 1660 ℃. Density: 4.51 g/cm 3 . Electronic configuration of the Titanium atom in ascending order of orbital energies: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 2. Electronic configuration of the Titanium ...
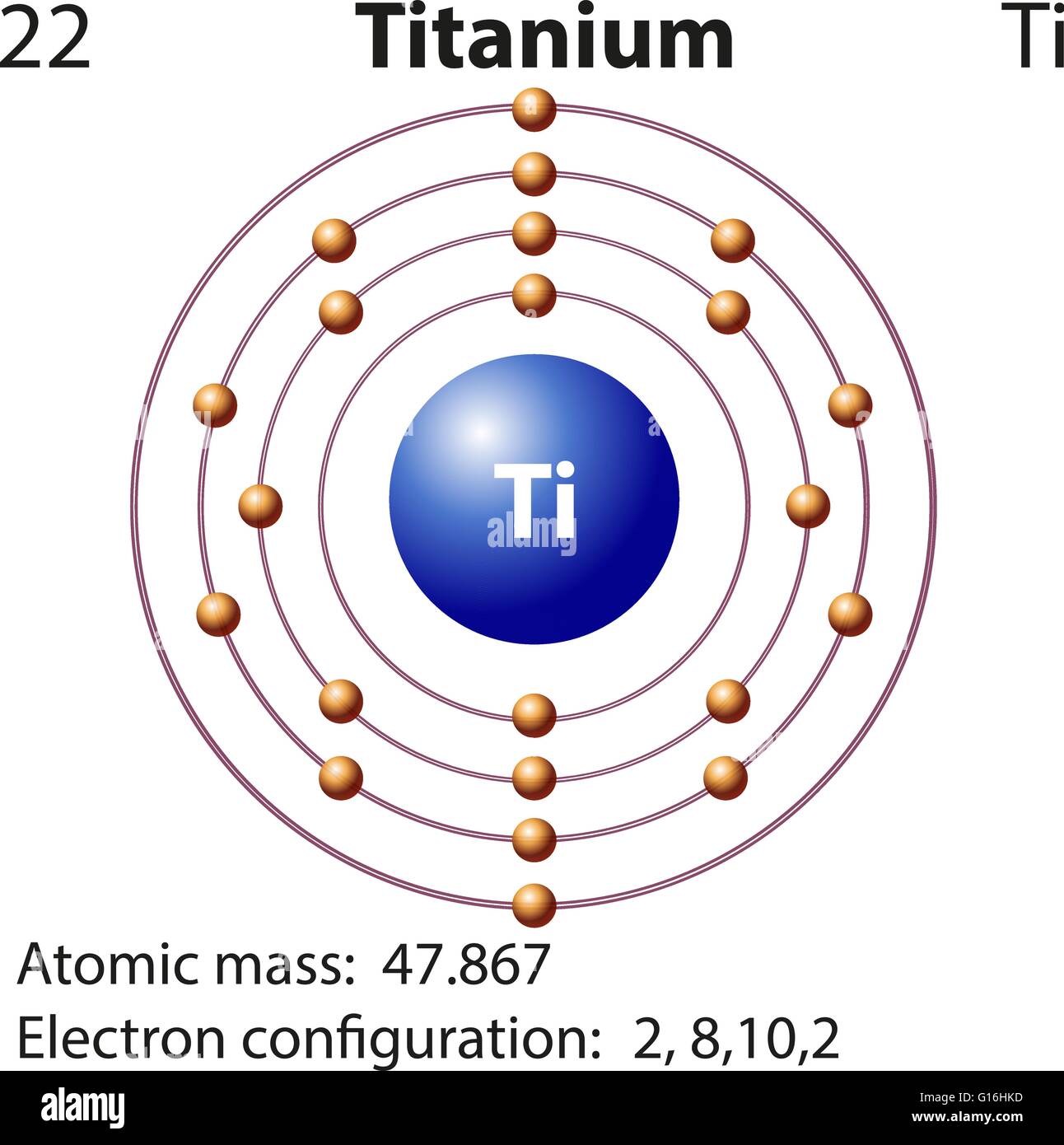
Titanium Bohr Diagram - schematron.org May 17, 2019 · For that, we have electron shell diagrams. Here are electron shell atom diagrams for the elements, ordered by increasing atomic number. For each electron shell atom diagram, the element symbol is listed in the nucleus. This is a Bohr Diagram of a titanium atom. The nucleus of a titanium atom has 22 protons and 26 neutrons.
Titanium(Ti) electron configuration and orbital diagram Scientist Niels Bohr was the first to give an idea of the atom’s orbit. He provided a model of the atom in 1913. The complete idea of the orbit is given there. The electrons of the atom revolve around the nucleus in a certain circular path. These circular paths are called orbit(shell). These orbits are expressed by n. [n = 1,2,3,4 . . . The serial number of the orbit] K is the name of the first orbit, L is the second, M is the third, N is the name of the fourth orbit. The electron holding capacity of each orbit is 2n2. For example, 1. n = 1 for K orbit. The electron holding capacity of K orbit is 2n2 = 2 × 12= 2 electrons. 2. For L orbit, n = 2. The electron holding capacity of the L orbit is 2n2 = 2 × 22= 8 electrons. 3. n=3 for M orbit. The maximum electron holding capacity in M orbit is 2n2 = 2 × 32 = 18 electrons. 4. n=4 for N orbit. The maximum electron holding capacity in N orbit is 2n2 = 2 × 42= 32 electrons. Therefore, the maximum electron holding capacity in the first shell...
PDF Molecular orbital diagram of ticl4 Molecular orbital diagram of ticl4 Titanium tetrachloride Names IUPAC Name Titanium (IV) Chloride Other Names Titanium Tetrachloride Tetrachlorotitanium Identifiers CAS Number 7550-45- Y 3D model (JSmol) Interactive image ChemSpider 22615 Y ECHA InfoCard 100.028,584 EC Number 231-441-9 MeSH
CH3OH (Methanol) Intermolecular Forces - Techiescientist 26.2.2022 · In O, the 2s and 2p orbitals also overlap to form hybrid orbitals. Two-hybrid orbitals contain lone pair, one overlaps with s orbital of hydrogen, and one of them overlaps with the sp 3 hybrid orbital of C. Methanol has a tetrahedral geometry as it is a molecule of AX 4 type where a central atom has four side atoms and no lone pairs.
Draw and explain the orbital diagram for titanium. | Study.com Draw and explain the orbital diagram for titanium. Transition Metals: There are, in total, four transition series of the d-block elements starting with 3d, 4d, 5d and 6d respectively.
Titanium (Ti) - ChemicalAid Titanium (Ti) has an atomic mass of 22. Find out about its chemical and physical properties, states, energy, electrons, oxidation and more.
SOLVED:Draw a crystal field energy-level diagram for the ... Energy level diagram. So, first off, all we discuss about the reluctantly configurations or titanium three plus is equal toe here. Argon and the street. Even that Minniti orbital contained one electron. And here argon is ating 18 plus 1 19 left contentment 1911 are involved in that in m three. Plus I'm. And for titanium, There is 20 to let Tom.
What is the Orbital notation for titanium? - Answers Orbital notation shows the number of electronics in an orbit. The orbital notation of Hydrogen is a circle with one slash through it. The electron configuration of Hydrogen is 1(s^1). Source: www ...
physical chemistry: bonding Flashcards - Quizlet Draw a diagram to show how two molecules of hydrogen fluoride are attracted to each other by the type of intermolecular force that you stated in part (d)(i ... (2+) requires loss of e- from a 2(p) orbital or 2nd energy level or2nd shell and Mg(2+) requires loss of e- from a 3(s) ... Titanium is also a strong material that has a high melting point.
Orbital Diagram of All Elements (Diagrams given Inside) Orbital diagrams (Orbital box diagrams) of all elements are mentioned in the chart given below. Orbital diagrams (Orbital box diagrams) of all elements are mentioned in the chart given below. ... Orbital diagram of Titanium (Ti) 23: Orbital diagram of Vanadium (V) 24: Orbital diagram of Chromium (Cr) 25: Orbital diagram of Manganese (Mn) 26 ...
What Is the Electron Configuration for Titanium? By looking at the electron configuration for titanium, it is possible to determine how its electrons are arranged. There are two electrons in the s orbital of the first energy level. The second level contains eight electrons, with two in the s orbital and six in the p orbital. There are electrons in three orbitals of the third energy level.
Ti3 electron configuration | electron configuration for ti ... Orbital Diagram of Titanium (Ti), electron configuration . Electronic configuration of Mn 2+ is [Ar] 18 3d 5. Electronic configuration of Fe 2+ is [Ar] 18 3d 6 . It is known that half-filled and fully-filled orbitals are more stable ; Electron Configuration: [Xe]4f145d96s1 Oxidation State: 2,4 Crystal Structure: cubic. Discovered by J. Scaliger ...
HCOOH Lewis Structure, Molecular Geometry, Hybridization ... 25.2.2022 · The orbital diagram of formic acid, which represents the sigma bonds, is shown below. We can observe from the orbital diagram is that the carbon atom is the sp2 hybridized and one of the oxygen atoms is also sp2 hybridized whereas another oxygen atom bonded to hydrogen and carbon atom, is sp3 hybridized.
Alveolar process - Wikipedia Terminology. The term alveolar ('hollow') refers to the cavities of the tooth sockets, known as dental alveoli. The alveolar process is also called the alveolar bone or alveolar ridge. The curved portion is referred to as the alveolar arch. The alveolar bone proper, also called bundle bone, directly surrounds the teeth. The term alveolar crest describes the extreme rim of the bone …







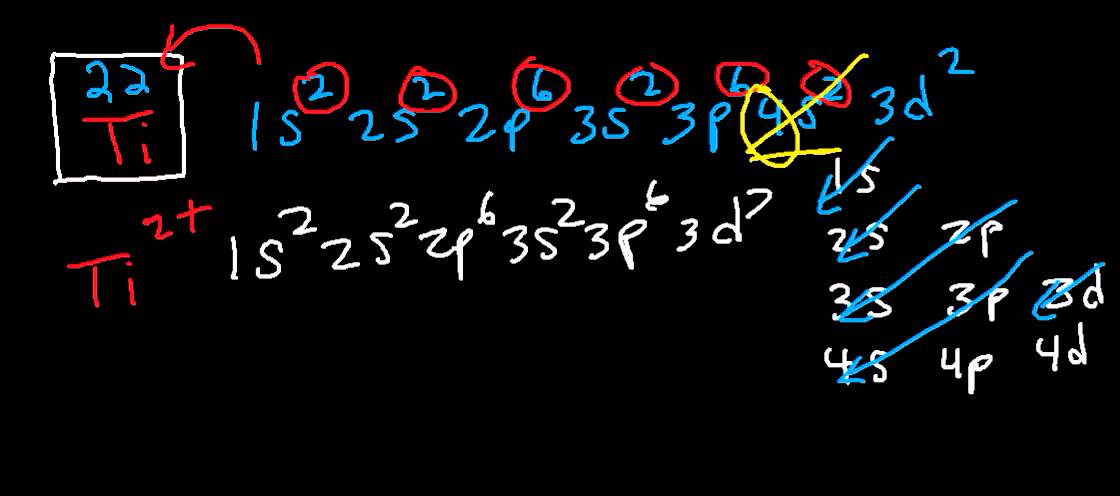














0 Response to "36 orbital diagram of titanium"
Post a Comment