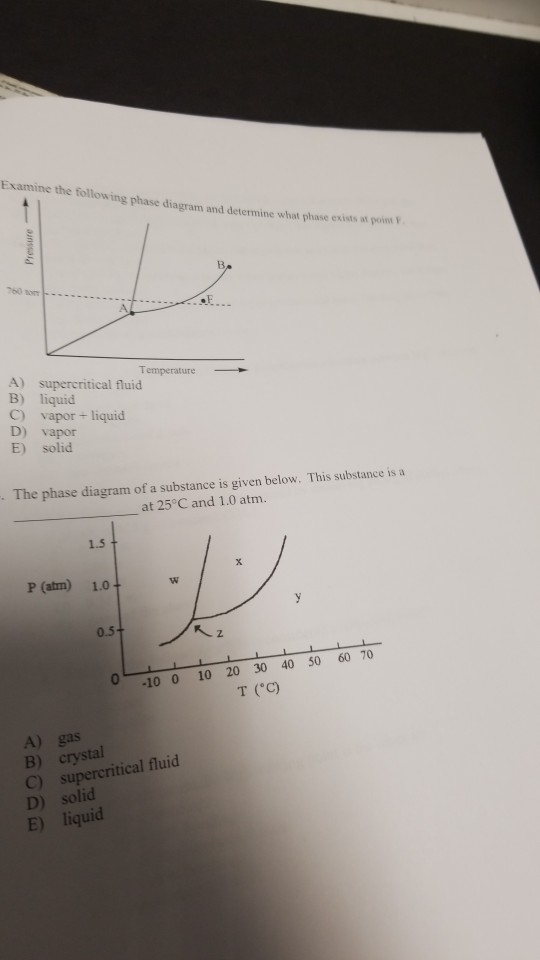
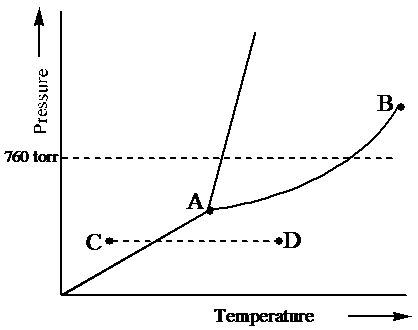
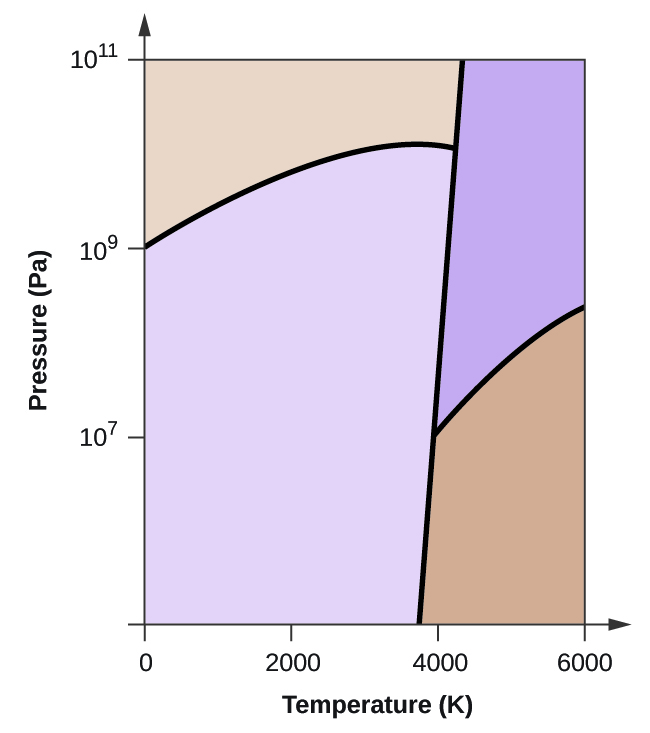
36 examine the following phase diagram and determine what phase exists at point c.
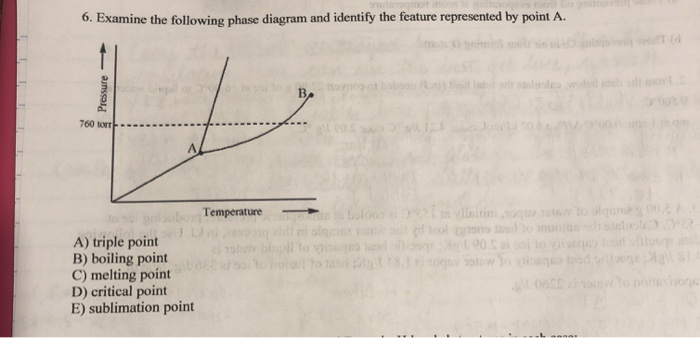
Examine the following phase diagram and id... | Clutch Prep Examine the following phase diagram and identify the feature represented by point B. A) Melting point. B) Triple point. C) Critical point. D) Sublimation point. E) Boiling point. Learn this topic by watching Phase Diagrams Concept Videos. Chapter 10/11/12 Terms Flashcards - Quizlet A) Ek (40°C) < Ek (20°C); Ep (40°C) ≈ Ep (20°C) B) Ek (40°C) > Ek (20°C); Ep (40°C) ≈ Ep (20°C) C) Ep (40°C) < Ep (20°C); Ek (40°C) ≈ Ek (20°C)
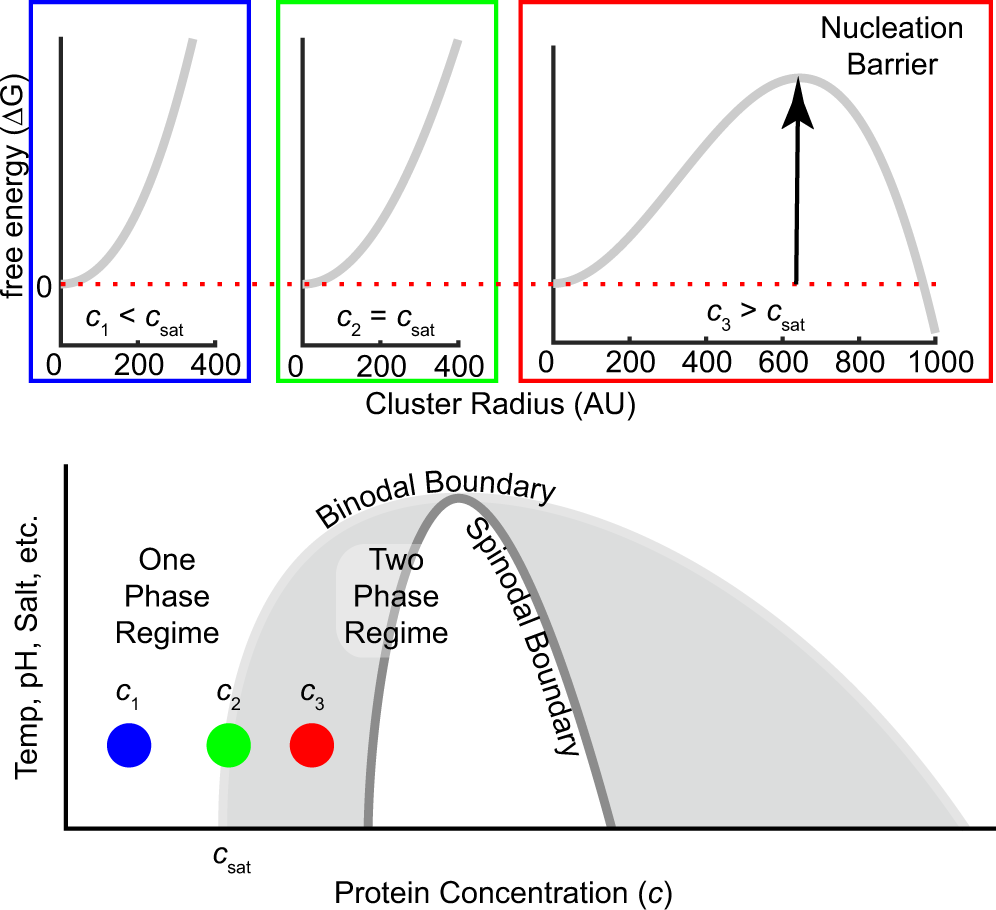
Ternary Phase Diagram - an overview | ScienceDirect Topics A point having composition (A, B, C) on Figure 4.23 can be located anywhere on the ternary diagram and such a point represents the overall composition of the phase, regardless of the number of phases. The compositions on a ternary diagram must sum to unity if in mole or weight fractions, or to 100 if in percent.
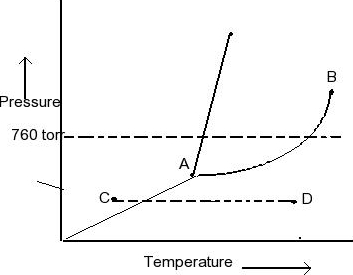
Examine the following phase diagram and determine what phase exists at point c.
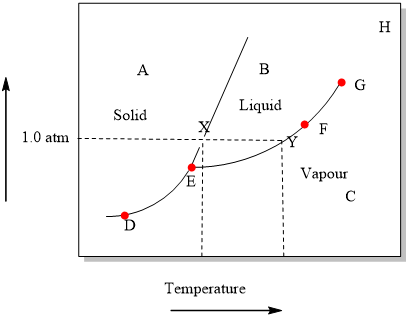
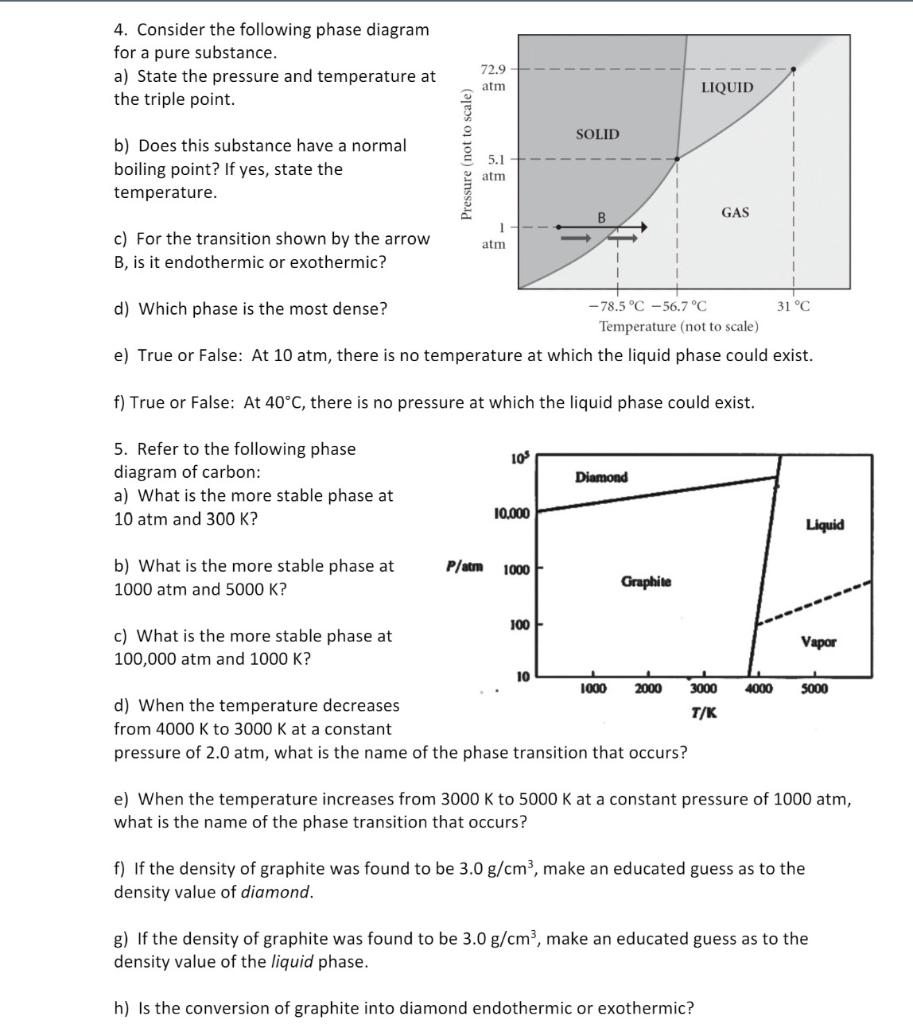
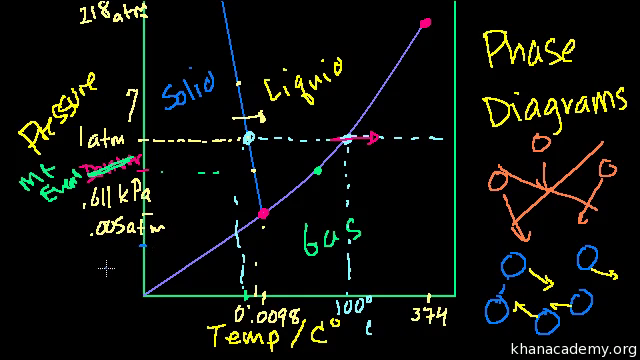
Phase Diagrams - Chemistry We can use the phase diagram to identify the physical state of a sample of water under specified conditions of pressure and temperature. For example, a pressure of 50 kPa and a temperature of −10 °C correspond to the region of the diagram labeled "ice.". Under these conditions, water exists only as a solid (ice). Best Chemistry 02 chapter 10/11/12 Terms Flashcards - Quizlet A) Ek (40°C) < Ek (20°C); Ep (40°C) ≈ Ep (20°C) B) Ek (40°C) > Ek (20°C); Ep (40°C) ≈ Ep (20°C) C) Ep (40°C) < Ep (20°C); Ek (40°C) ≈ Ek (20°C) PDF CHAPTER 9 PHASE DIAGRAMS PROBLEM SOLUTIONS ε and phases ... mass fraction of each phase is 0.5, estimate: (a) The temperature of the alloy (b) The compositions of the two phases Solution (a) We are given that the mass fractions of α and liquid phases are both 0.5 for a 30 wt% Sn-70 wt% Pb alloy and asked to estimate the temperature of the alloy. Using the appropriate phase diagram, Figure 9.8, by trial
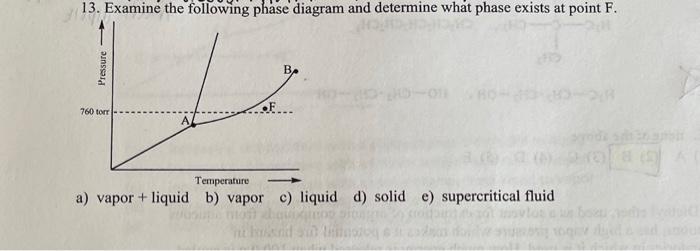
Examine the following phase diagram and determine what phase exists at point c.. Solved 12) Examine the following phase diagram and ... 100% (1 rating) Transcribed image text: 12) Examine the following phase diagram and identify the feature represented by point A. 760 torr Temperature A) melting point B) critical point C) triple point D) sublimation point E) boiling point 13) In hydrogen iodide are the most important intermolecular forces A) B) C) dipole-dipole forces London dispersion forces hydrogen bonding D) E) covalent bonds polar covalent bonds 14) Which of the following substances will have hydrogen bonds between ... Features of Phase Diagrams (M11Q1) - UW-Madison Chemistry ... Using the phase diagram for water, we can determine that the state of water at each temperature and pressure given are as follows: (a) solid; (b) liquid; (c) liquid; (d) gas; (e) solid; (f) gas. Check Your Learning Examine the following phase diagram and determine what ... Examine the following phase diagram and determine what phase exists at point F. Examine the following phase diagram and determine what phase exists at point F. A)Vapor + Liquid. B)Vapor. C)Liquid. D)Solid. E)Supercritical fluid. Categories. PDF Chapter 9: Phase Diagrams - Florida International University TA A 35 Co 32 CL At TA = 1320°C: Only Liquid ( L) CL = C o ( = 35 wt% Ni) At TB = 1250°C: Both α and L CL = Cliquidus ( = 32 wt% Ni here) Cα = Csolidus ( = 43 wt% Ni here) At TD = 1190°C: Only Solid ( α) Cα = Co ( = 35 wt% Ni ) Co = 35 wt% Ni. Adapted from Fig. 9.3(b), Callister 7e.
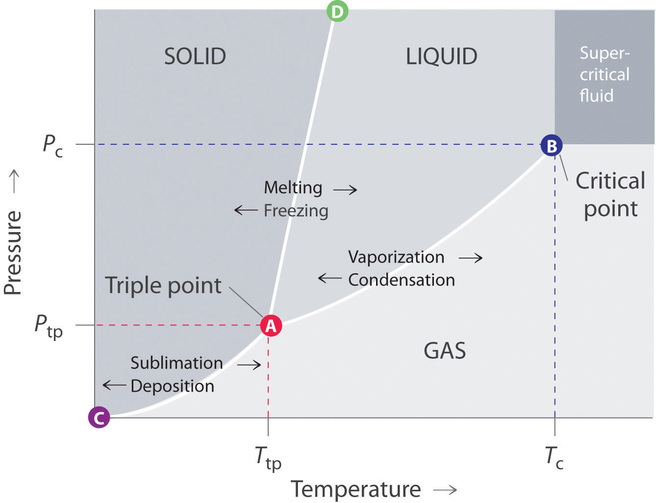
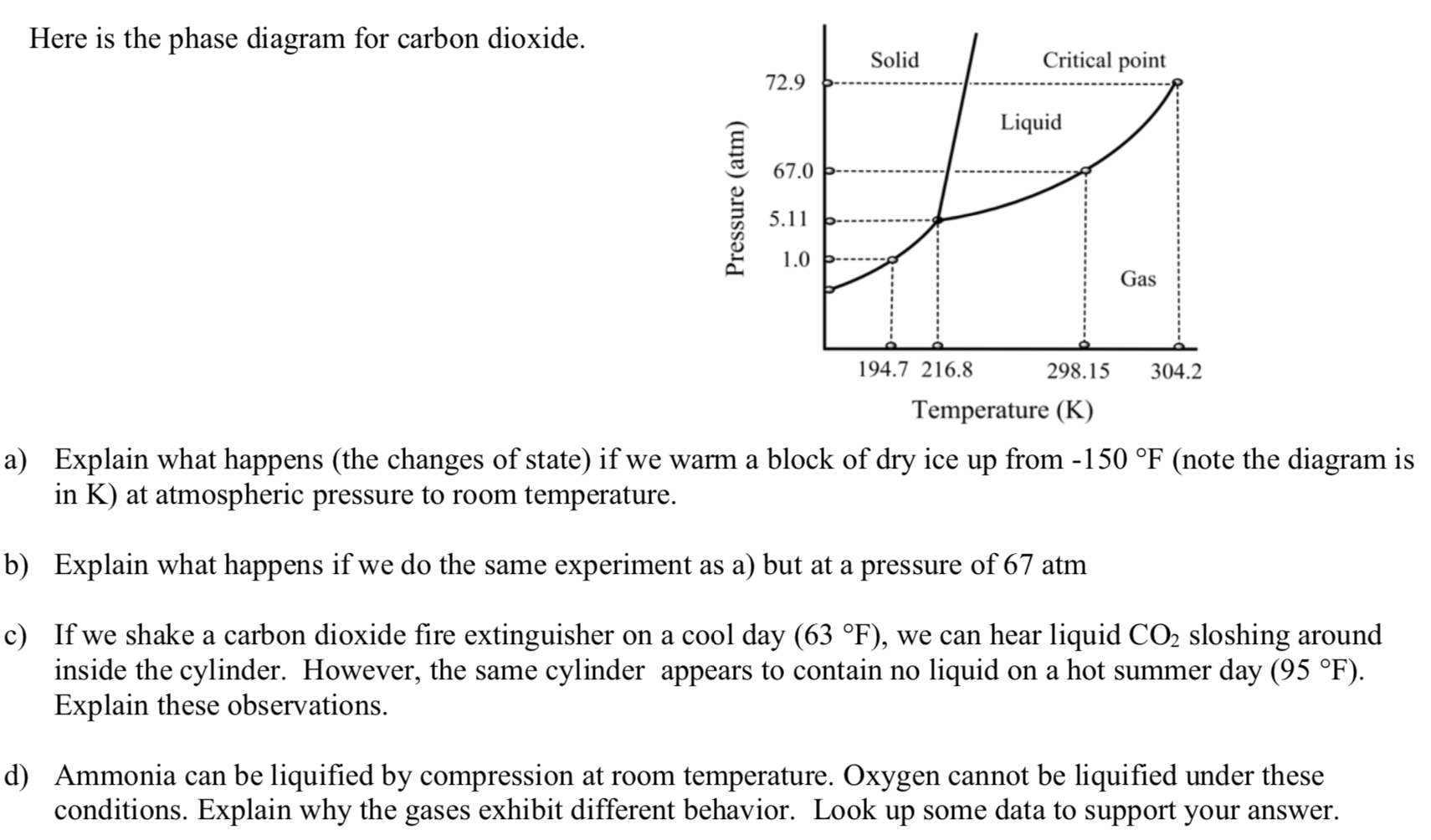
Solved Examine the following phase diagram and determine ... Examine the following phase diagram and determine what phase exists at point C. 1. gas and liquid. 2. gas. 3. liquid. 4. solid. 5. supercritical fluid Sample Questions - Chapter 13 - Texas A&M University (a) 1, 2, and 3 (b) 4, 5, and 6 (c) 1 and 2 only (d) 4 and 6 only (e) some other combination 11. According to the phase diagram given for Compound Y, what description is correct? (a) At the temperature and pressure at point 4, Y(g) will spontaneously convert to Y(l).(b) At 0 o C and 1200 torr, Y exists as a solid. (c) At the pressure and temperature of point 1, Y(s) will spontaneously convert ... Solved 13. Examine the following phase diagram and ... Solved 13. Examine the following phase diagram and determine | Chegg.com. Science. Chemistry. Chemistry questions and answers. 13. Examine the following phase diagram and determine what phase exists at point F. Pressure В. 760 torr Temperature a) vapor + liquid b) vapor c) liquid d) solid e) supercritical fluid. Question: 13. Phase Diagrams | Chemistry for Majors - Lumen Learning Consider the phase diagram for carbon dioxide shown in Figure 5 as another example. The solid-liquid curve exhibits a positive slope, indicating that the melting point for CO 2 increases with pressure as it does for most substances (water being a notable exception as described previously). Notice that the triple point is well above 1 atm, indicating that carbon dioxide cannot exist as a liquid ...
PDF Lecture Notes 2: Physical Equilibria - Phase Diagrams The diagram you mostly find associated with different phases of a substance is the so-called "phase diagram". This diagram shows the regions of stability of different phases as a function of temperature and pressure. The interfaces between these regions will be the phase transition lines. The phase diagram for CO 2 is shown below. States of Matter Review | States of Matter Quiz - Quizizz 60 seconds. Q. In an experiment, a scientist burned 10.0 g of methane, and the temperature of the 500.0 mL of water in the calorimeter changed from 21.0°C to 26.6°C. The specific heat of water is 4.18 J/g·°C. How much energy was released in joules? answer choices. 5.00 x 10^3 J. Chem Test #4 Flashcards | Quizlet Examine the following phase diagram and determine what phase exists at point F Vapor When the electron cloud of a molecule is easily distorted, the molecule has a high _____________. 10.4 Phase Diagrams - Chemistry Elemental carbon has one gas phase, one liquid phase, and two different solid phases, as shown in the phase diagram: (a) On the phase diagram, label the gas and liquid regions. (b) Graphite is the most stable phase of carbon at normal conditions. On the phase diagram, label the graphite phase.
PDF 1331 - Chapter 11 Questions Ignore Questions 3, 10, 37 ... (A) 10.1 kJ (B) 13.1 kJ (C) 16.1 kJ (D) 48.6 kJ 38. Examine the following phase diagram and identify the feature represented by point A. A. melting point B. critical point C. triple point D. sublimation point 39. What are the changes in phase going from points A to B to C to D A B D C T P
phase diagrams of pure substances - chemguide Phase diagrams. A phase diagram lets you work out exactly what phases are present at any given temperature and pressure. In the cases we'll be looking at on this page, the phases will simply be the solid, liquid or vapour (gas) states of a pure substance. This is the phase diagram for a typical pure substance.
PDF Determination of the Solid-Liquid Phase Diagram for ... • Phase Diagrams Phase diagrams are graphs that give information on the equilibrium temperature and pressure for a particular compound. The equilibria occur for the solid- liquid plateau, liquid-vapor plateau and solid-vapor plateau. In this experiment, the phase diagram is shown for the solid-liquid equilibrium point, and varies from 100% ...
M11Q1: Features of Phase Diagrams - Chem 103/104 Resource Book Using the phase diagram for water, we can determine that the state of water at each temperature and pressure given are as follows: (a) solid; (b) liquid; (c) liquid; (d) gas; (e) solid; (f) gas. Check Your Learning
DOCX Cabarrus County Schools / District Homepage Examine the phase diagram of carbon dioxide. ... According to the phase diagram below, a sample of water exists in two phases at a pressure of 1 atm and a temperature of 0°C. ... At the top of Mount Everest, water boils at about 72°C, which is 28°C lower than the boiling point at sea level. Why is the boiling point of water lower at the top ...
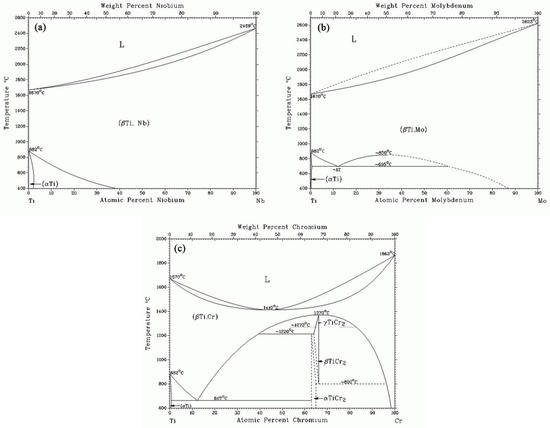
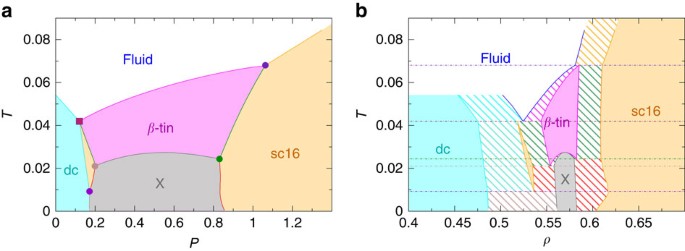
PDF Teach Yourself Phase Diagrams and Phase Transformations as axes are called phase diagrams. DEF. A phase diagram (or equilibrium diagram) is a diagram with T and composition as axes, showing the equilibrium constitution. The phase diagram of an alloy made of components A and B, for all combinations of T and X B, defines the A-B system. Binary systems have two components, ternary systems three, and so on.
Chemistry Chapter 12 Flashcards - Quizlet Examine the following phase diagram and identify the feature represented by point A A) melting point ... Examine the following phase diagram and determine what phase exists at point F. A) vapor+liquid B) vapor C) liquid D) solid E) supercritical fluid. B.
Solved Examine the following phase diagram and determine ... Question: Examine the following phase diagram and determine what phase exists at point 760 som Temperature A) supercritical fluid B) liquid C) vapor+liquid D) vapor E) solid The phase diagram of a substance i s given below. This substance is a at 25°C and 1.0 atm. 1.5 P (atm) 1.0 0.5T -10 0 10 20 30 40 50 60 70 T ('C) A) gas B) crystal C ...
HQ 12 - Chapter 12 -Consider the following phase diagram ... Chapter 12-Consider the following phase diagram and identify the process occurring as one goes from point C to point D. Increasing temperature with a phase change from solid to vapor -Examine the following phase diagram and determine what phase exists at point F.
CHEM Exam 6 Flashcards | Quizlet 5) The phase diagram for xenon has a solid- liquid curve with a positive slope. Which of the following is true? A) Solid xenon has a higher density than liquid xenon. B) Solid xenon has the same density as liquid xenon. C) The phase diagram cannot be used to predict which phase of xenon is denser. D) Freezing xenon is an endothermic process.
PDF Lecture 19: 11.23.05 Binary phase diagrams 3-Dimensional Depiction of Temperature-Composition Phase Diagram of Bismuth, Tin, and Lead at 1atm. The diagram has been simplified by omission of the regions of solid solubility. Each face of the triangular a eutectic. There is also a peritectic point in the Bi-Pb phase diagram. Figure by MIT OCW.
PDF CHAPTER 9 PHASE DIAGRAMS PROBLEM SOLUTIONS ε and phases ... mass fraction of each phase is 0.5, estimate: (a) The temperature of the alloy (b) The compositions of the two phases Solution (a) We are given that the mass fractions of α and liquid phases are both 0.5 for a 30 wt% Sn-70 wt% Pb alloy and asked to estimate the temperature of the alloy. Using the appropriate phase diagram, Figure 9.8, by trial
Best Chemistry 02 chapter 10/11/12 Terms Flashcards - Quizlet A) Ek (40°C) < Ek (20°C); Ep (40°C) ≈ Ep (20°C) B) Ek (40°C) > Ek (20°C); Ep (40°C) ≈ Ep (20°C) C) Ep (40°C) < Ep (20°C); Ek (40°C) ≈ Ek (20°C)
Phase Diagrams - Chemistry We can use the phase diagram to identify the physical state of a sample of water under specified conditions of pressure and temperature. For example, a pressure of 50 kPa and a temperature of −10 °C correspond to the region of the diagram labeled "ice.". Under these conditions, water exists only as a solid (ice).







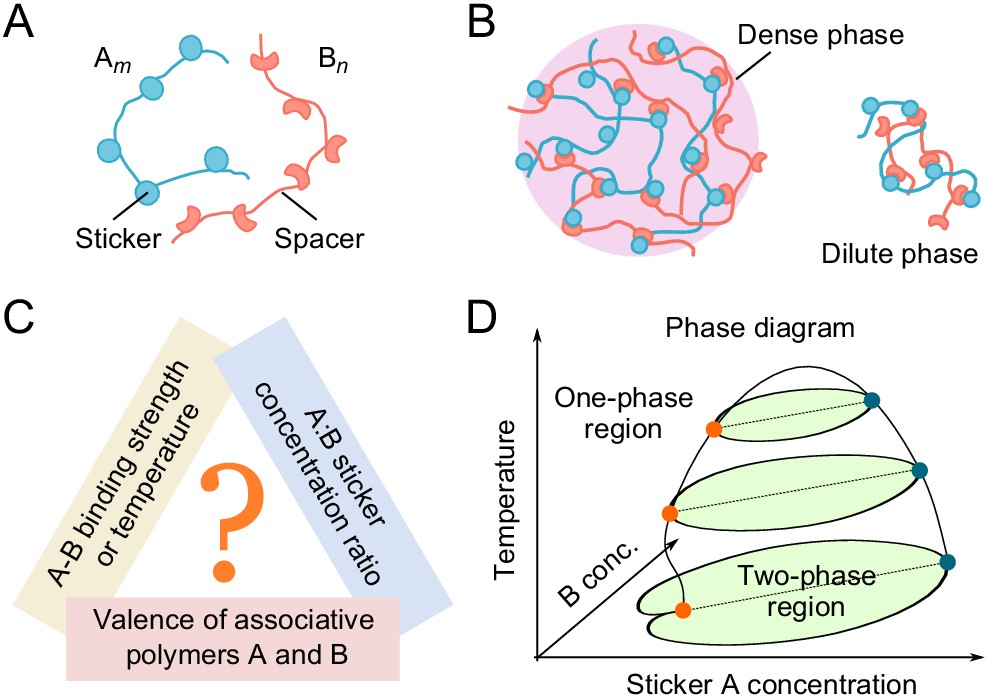

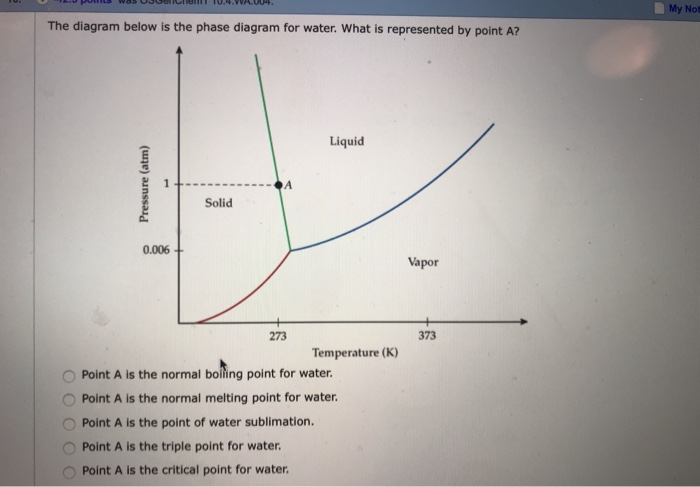


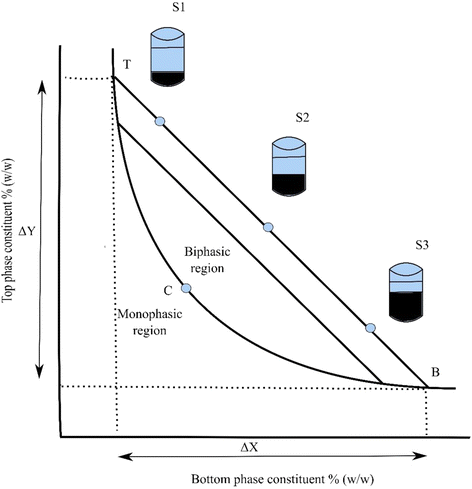







0 Response to "36 examine the following phase diagram and determine what phase exists at point c."
Post a Comment