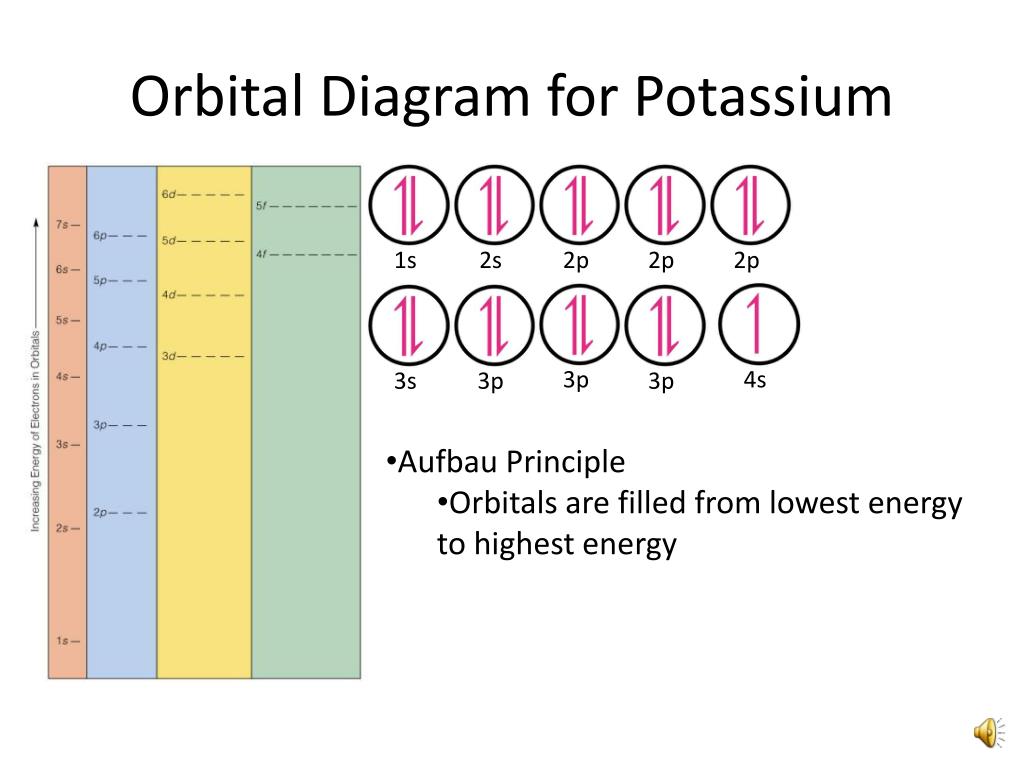
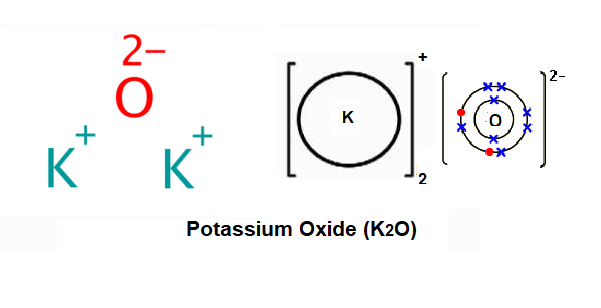
37 Orbital Diagram For Potassium
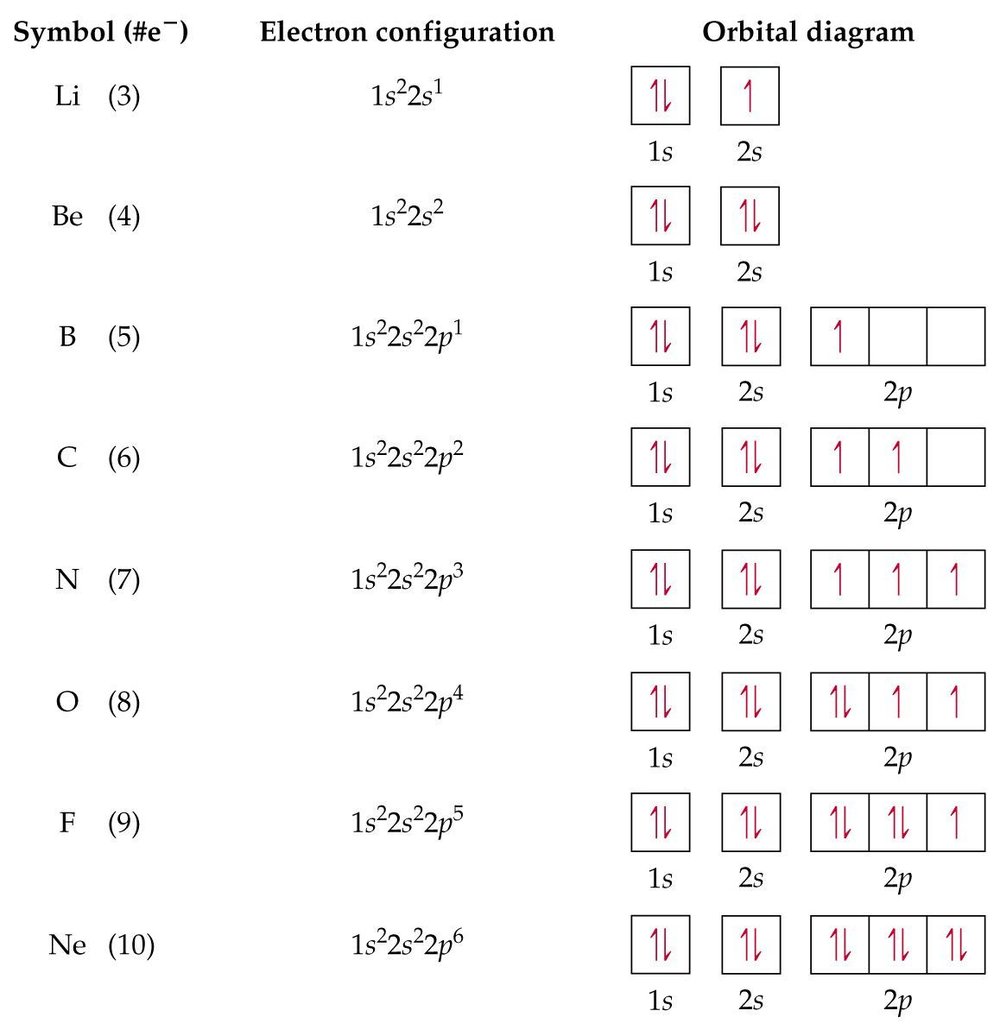
Solved What is the likely formula of potassium in the gas ... What is the likely formula of potassium in the gas phase? Draw a molecular orbital diagram to show your reasoning. Question: What is the likely formula of potassium in the gas phase? Draw a molecular orbital diagram to show your reasoning. Orbital Diagrams Chemistry Tutorial - AUS-e-TUTE for ... orbital diagram (orbital box diagram) : Pairs of electrons occupy the 1s, 2s, 2p x, 2p y, 2p z, 3s, 3p x, 3p y, 3p z, 4s orbital and two of the 3d orbitals, with only 1 electron occupying each of the other 3d orbitals and these electrons have parallel spin (arrows pointing in the same direction) in accordance with Hund's Rule.
File:Orbital representation diagram potassium - small.svg ... English: Orbital representation diagram depicting each orbital of an atom having its own circle within each sublevel. This diagram is for potassium. Date. 22 February 2010. Source. File:High School Chemistry.pdf, page 355. Author. CK-12 Foundation (raster), Adrignola (vector) SVG development.

Orbital diagram for potassium
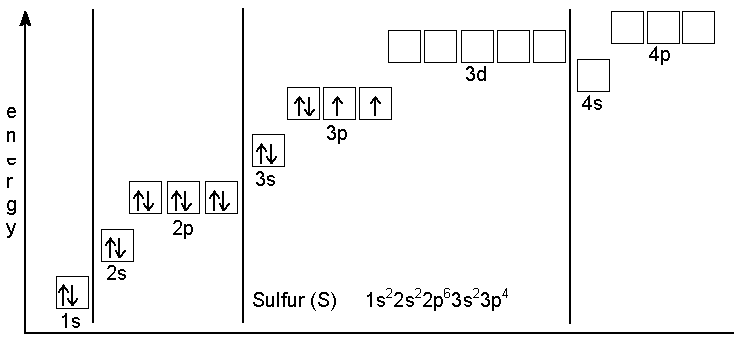
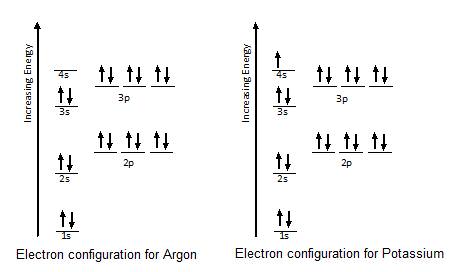
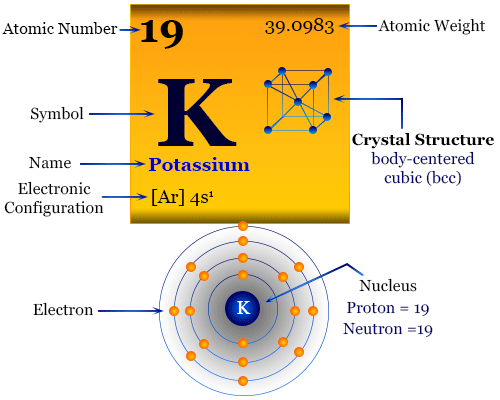
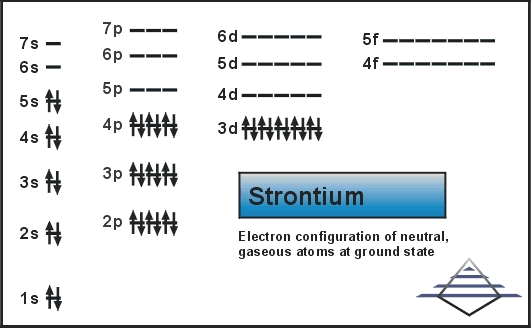
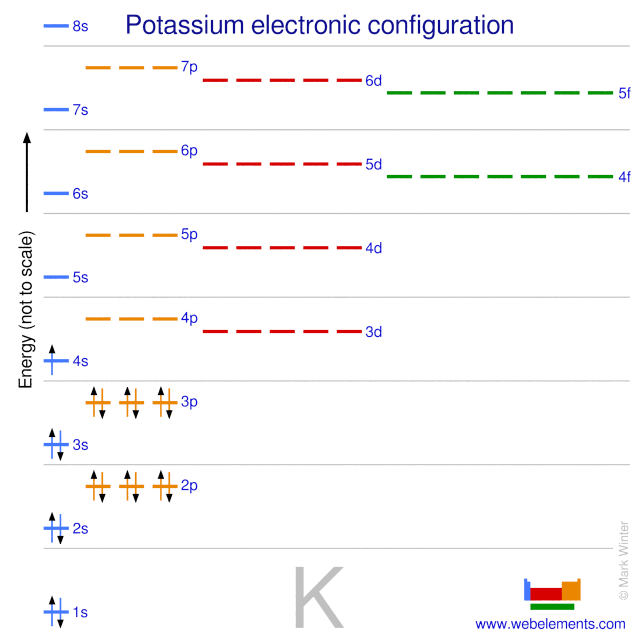
What are the electron configuration, orbital diagram, and ... So the electron configuration of potassium will involve 19 electrons. The full electron configuration of potassium is 1s2 2s2 2p6 3s2 3p6 4s1. The noble gas notation is [Ar]4s1. The orbital diagram shows the increase in energy from one energy sublevel to the next. Orbital diagram for potassium? - Answers The bohr diagram is the diagram of the electrons on the orbital layers of the nucleus of an atom. for potassium, you would put 2 electrons on the first layer, 8 on the second layer, and 9 on the ... How to Write the Orbital Diagram for Potassium (K) - YouTube To write the orbital diagram for the Potassium atom (K) first we need to write the electron configuration for just K. To do that we need to find the number o...
Orbital diagram for potassium. Electron Configuration (Section 5.2) - Surry County Public ... •Potassium –Atomic Number = 19 –1s22s 22p63s 3p64s1 –[Ar]4s1. Pauli Exclusion Principle •Two electrons in same orbital have different spins . ... •The orbital diagram below violates Hund’s rule because the third electron does not enter the empty 2p orbital Benchmark Alert!! Quadruple bond - Wikipedia History. Chromium(II) acetate, Cr 2 (μ-O 2 CCH 3) 4 (H 2 O) 2, was the first chemical compound containing a quadruple bond to be synthesized. It was described in 1844 by E. Peligot, although its distinctive bonding was not recognized for more than a century.. The first crystallographic study of a compound with a quadruple bond was provided by Soviet chemists for salts of Re Orbital Diagram For Nitrogen (N) | Nitrogen Electron ... Feb 15, 2021 · Orbital Diagram For Nitrogen (N) | Nitrogen Electron Configuration February 15, 2021 by Sneha Leave a Comment Nitrogen Electron Configuration : When we talk about school subjects, then one of the major subjects which are very important for knowledge perspective is science . topblogtenz.com › cyanide-cn-lewis-structureCN- lewis structure, molecular orbital diagram, and, bond order In this article, we will study the Cyanide (CN-) lewis structure, molecular orbital diagram(MO), its bond order, formal charges, and hybridization. Cyanide can be a colorless gas in the form of hydrogen cyanide, sodium cyanide, potassium cyanide, etc.
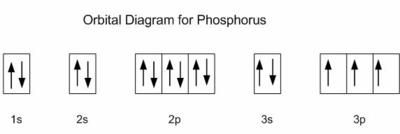
How to Do Orbital Diagrams - Sciencing Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics. valenceelectrons.com › oxygen-electron-configurationOxygen(O) electron configuration and orbital diagram Oxygen(O) is the 8th element in the periodic table and its symbol is ‘O’. This article gives an idea about the electron configuration of oxygen and orbital diagram, period and groups, valency and valence electrons of oxygen, bond formation, compound formation, application of different principles. Arrangements of electrons in the orbitals of an atom is ... Chemically potassium behaves like sodium, as an alkali metal. It appears the next electron is in an s orbital, not a 'd' orbital. It turns out the energy of the 4s orbital is very close to the energy of the 3d orbital at potassium. But the energy of the 4s orbital is lower in energy compared to the 3d. Potassium(K) electron configuration and orbital diagram To write the orbital diagram of potassium (K), you have to do the electron configuration of potassium. Which has been discussed in detail above. 1s is the closest and lowest energy orbital to the nucleus. Therefore, the electron will first enter the 1s orbital.
Fluorine(F) electron configuration and orbital diagram Fluorine(F) is the 9th element in the periodic table and its symbol is ‘F’. This article gives an idea about the electron configuration of fluorine and orbital diagram, period and groups, valency and valence electrons of fluorine, bond formation, compound formation, application of different principles.Hopefully, after reading this article you will know in detail about this. valenceelectrons.com › hydrogen-electron-configurationHydrogen(H) electron configuration and orbital diagram Hydrogen(H) is the 1st element in the periodic table and its symbol is ‘H’. This article gives an idea about the electron configuration of hydrogen, period and groups, valency and valence electrons of hydrogen, bond formation, compound formation, application of different principles. What are the electron configuration, orbital diagram, and ... So the electron configuration of potassium will involve 19 electrons. The full electron configuration of potassium is 1s2 2s2 2p6 3s2 3p6 4s1. The noble gas notation is [Ar]4s1. The orbital diagram shows the increase in energy from one energy sublevel to the next. File:Orbital representation diagram potassium.svg ... Orbital representation diagram potassium.svg. English: Orbital representation diagram depicting each orbital of an atom having its own circle within each sublevel. This diagram is for potassium. Date. 22 February 2010. Source. File:High School Chemistry.pdf, page 355. Author.
Answered: What is the likely formula of potassium… | bartleby Browse 5+ million homework and textbook solutions, concept explainers, videos and more. Search concepts or drop in your homework problem! Our library grows every minute-keep searching! Science Chemistry Q&A Library What is the likely formula of potassium in the gas phase? Draw a molecular orbital diagram and explain.
Neon(Ne) electron configuration and orbital diagram Neon(Ne) is the 10th element in the periodic table and its symbol is ‘Ne’. This article gives an idea about the electron configuration of neon and orbital diagram, period and groups, valency and valence electrons of neon, application of different principles.
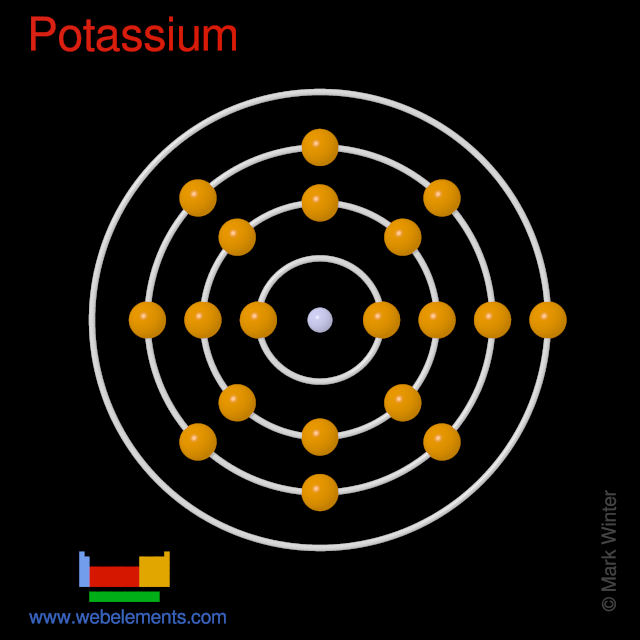
Orbital Diagram For Potassium — UNTPIK APPS orbital diagram for potassium qaswers the bohr diagram is the diagram of the electrons on the orbital layers of the nucleus of an atom for potassium you would put 2 electrons on the first layer 8 on the second … layer and 9 on the third layer this is because the atomic number of potassium k is 19 therefore has 19 protons and 19 electrons
PDF Electron Configuration and Orbital Filling Diagram WS Key Electron Configuration & Orbital Filling Diagram WS Usinq the Lonq Method, qive the Electron Configuration: Magnesium (Mg): Potassium (K): Lithium (Li): Nickel (Ni): Identi the followin Elements: Is2 2s2 2p2: 1s2 2s2 2p6. [Arl 4s2 3d10 4p5 Órbital Fillinq Diaqrams Is 2s 2P is the element 3p 12. c 13. Ni (short cut) Identifv the followinq elements:
periodictableguide.com › orbital-diagram-of-allOrbital Diagram of All Elements (Diagrams given Inside) Apr 10, 2021 · Orbital diagram of Phosphorus (P) 16: Orbital diagram of Sulfur (S) 17: Orbital diagram of Chlorine (Cl) 18: Orbital diagram of Argon (Ar) 19: Orbital diagram of Potassium (K) 20: Orbital diagram of Calcium (Ca) 21: Orbital diagram of Scandium (Sc) 22: Orbital diagram of Titanium (Ti) 23: Orbital diagram of Vanadium (V) 24: Orbital diagram of ...
Nitrogen(N) electron configuration and orbital diagram Orbital Diagram for Nitrogen (N) Nitrogen(N) excited state electron configuration. Atoms can jump from one orbital to another by excited state. This is called quantum jump. Ground state electron configuration of nitrogen is 1s 2 2s 2 2p 3. The p-orbital has three sub-orbitals. The sub-orbitals are p x, p y, and p z.
Soyuz (spacecraft) - Wikipedia Soyuz (Russian: Союз, IPA: , lit. 'Union') is a series of spacecraft which has been in service since the 1960s, having made more than 140 flights. It was designed for the Soviet space program by the Korolev Design Bureau (now Energia).The Soyuz succeeded the Voskhod spacecraft and was originally built as part of the Soviet crewed lunar programs.
Titanium(Ti) electron configuration and orbital diagram Orbital diagram for titanium(Ti) Titanium(Ti) excited state electron configuration. Atoms can jump from one orbital to another orbital by excited state. This is called quantum jump. Ground state electron configuration of titanium is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 2 4s 2. The valency of the element is determined by electron configuration in the ...
potassium superoxide KO2 chemistry - Creighton University Potassium superoxide is paramagnetic. The simple explanation is that with a formula of O2-2, there are simply an odd number of electrons in the anion (6 e-+ 6 e-+ 1 e-= 13 e-), hence it is paramagnetic. From a molecular orbital explanation, the peroxide ion has a molecular orbital configuration of (sigma)s 2 (sigma*)s 2 (sigma)p 4 (pi) 4 3 (pi ...
K+ Electron Configuration (Potassium Ion) - YouTube In this video we will write the electron configuration for K+, the Potassium ion. We'll also look at why Potassium forms a 1+ ion and how the electron config...
What is the electron configuration, orbital diagram, and ... The full electron configuration of potassium is 1s22s22p63s23p64s1. The noble gas notation is [Ar]4s1. The following orbital diagram shows the increase in energy from one energy sublevel to the next, but you can write them on the same level horizontally, Answer link
Potassium Electron Configuration (K) with Orbital Diagram Potassium Electron Configuration (K) with Orbital Diagram. Potassium Electron Configuration: Potassium is a chemical element. Its symbol is K that is taken from Neo-Latin kalium. The atomic number of potassium is 19. Firstly it was isolated from potash and the ashes of plants, from where its name is derived. In the periodic table.
Electron Configuration for Potassium (K) In writing the electron configuration for Potassium the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Potassium go in the 2s orbital. The next six electrons will go in the 2p orbital. The p orbital can hold up to six electrons.
(PDF) Clayden Organic Chemistry (1) | angie ... - Academia.edu Academia.edu is a platform for academics to share research papers.
SOLVED:Write an orbital diagram for the ground state of ... So here's the abbreviated um electron configuration for potassium. From there we can draw. The orbital diagram will keep A. R. As is, and then we have for us, and we have one electronic put an arbitrary up spin, and all the electrons within the noble gas core Oregon are going to be paired up because all those orbital's are full.
Free Printable Periodic Table of Elements Charts [Download] How To Read an Element on the Periodic Table. it is said the if a child understands how to stand than sure he will be able to walk slowly, similarly, if you come to know that how to read an element of a table than the rest 117 elements will be very easy for you read, hence last but not the least we will help you to read all the elements along with the name.
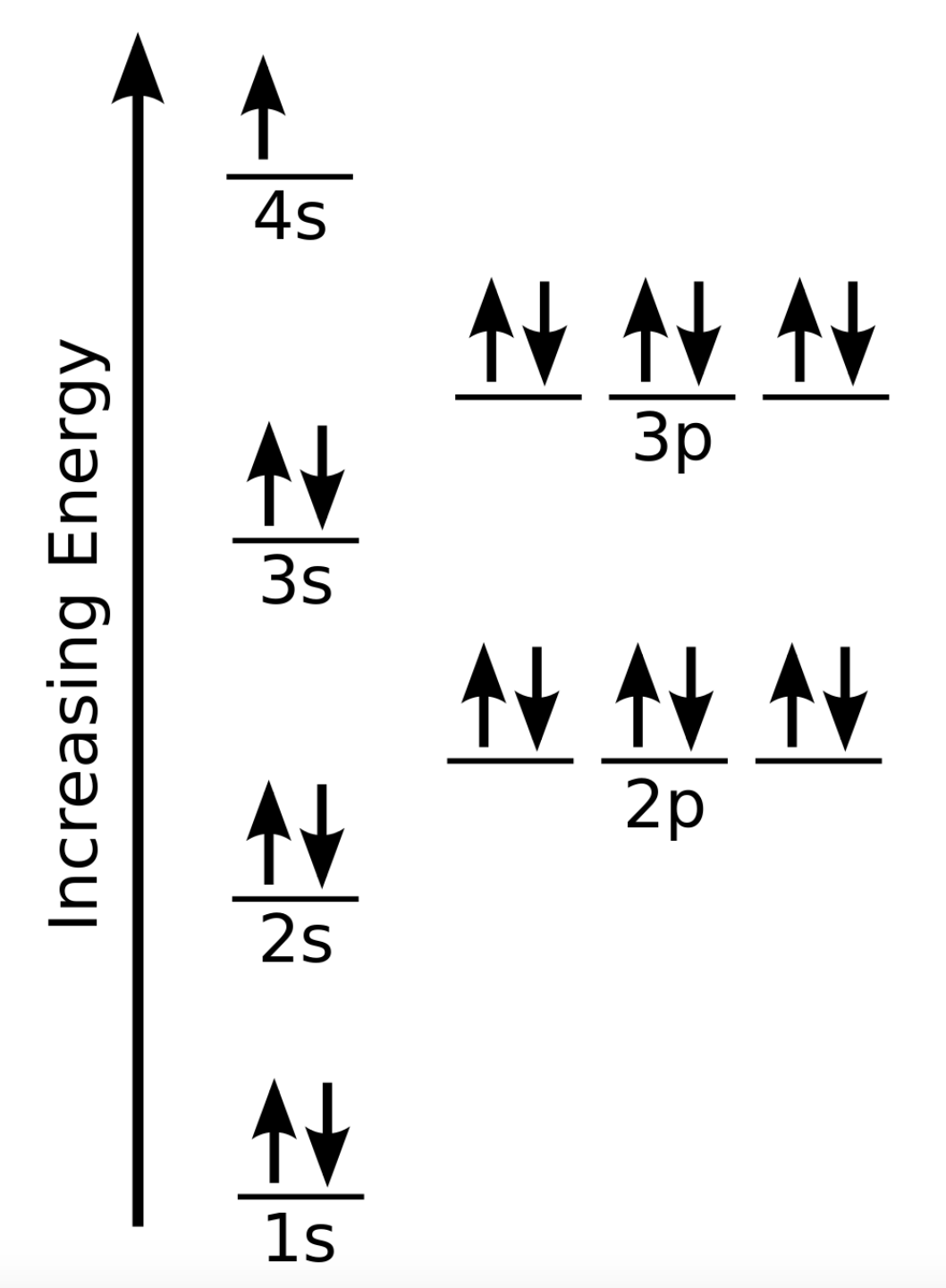
PDF Chapter 8 potassium (K; Z = 19) (b) technetium (Tc; Z = 43) (c) lead (Pb; Z = 82) PLAN: The atomic number gives the number of electrons, and the periodic table shows the order for filling orbitals. The partial orbital diagram includes all electrons added after the previous noble gas except those in filled inner sublevels.
chemistrygod.com › aufbau-principleAufbau Principle with Exceptions - ChemistryGod Feb 18, 2020 · From the previous table, the order of orbitals concurs with the order of the aufbau principle in the diagram. The energy of the orbital increases with n + l. Whenever there is a tie in the value, the energy increases with n. In the diagram, each orbital along the diagonal has the same value of n + l (see below).
Orbital Diagram For Barium - schematron.org Orbital Diagram For Barium. Step by Step: Electron Configurations and Electron Orbital Diagrams we need to count each box going from Hydrogen (#1) to Barium (#56), including Barium. The first two groups (columns) of the periodic table represent the 's' orbital group. This means that the s,p,d,f electron configuration for Barium.
How to Write the Orbital Diagram for Potassium (K) - YouTube To write the orbital diagram for the Potassium atom (K) first we need to write the electron configuration for just K. To do that we need to find the number o...
Orbital diagram for potassium? - Answers The bohr diagram is the diagram of the electrons on the orbital layers of the nucleus of an atom. for potassium, you would put 2 electrons on the first layer, 8 on the second layer, and 9 on the ...
What are the electron configuration, orbital diagram, and ... So the electron configuration of potassium will involve 19 electrons. The full electron configuration of potassium is 1s2 2s2 2p6 3s2 3p6 4s1. The noble gas notation is [Ar]4s1. The orbital diagram shows the increase in energy from one energy sublevel to the next.




















0 Response to "37 Orbital Diagram For Potassium"
Post a Comment