40 draw an electron dot diagram for ammonia
The molecule containing a triple covalent bond is : · The number of valence-shell bonding electron-dot model for HN3 is: · Draw an electron dot diagram to show ...1 answer · Top answer: Step 1: Find valence e - for all atoms. Add them together. N - 5 H - 1x3 = 3 Total = 8 Step2: Find octet e - for each atom and add them together.N - 8 H ... If you have a link or image of one you can send to me that would be appreciated!
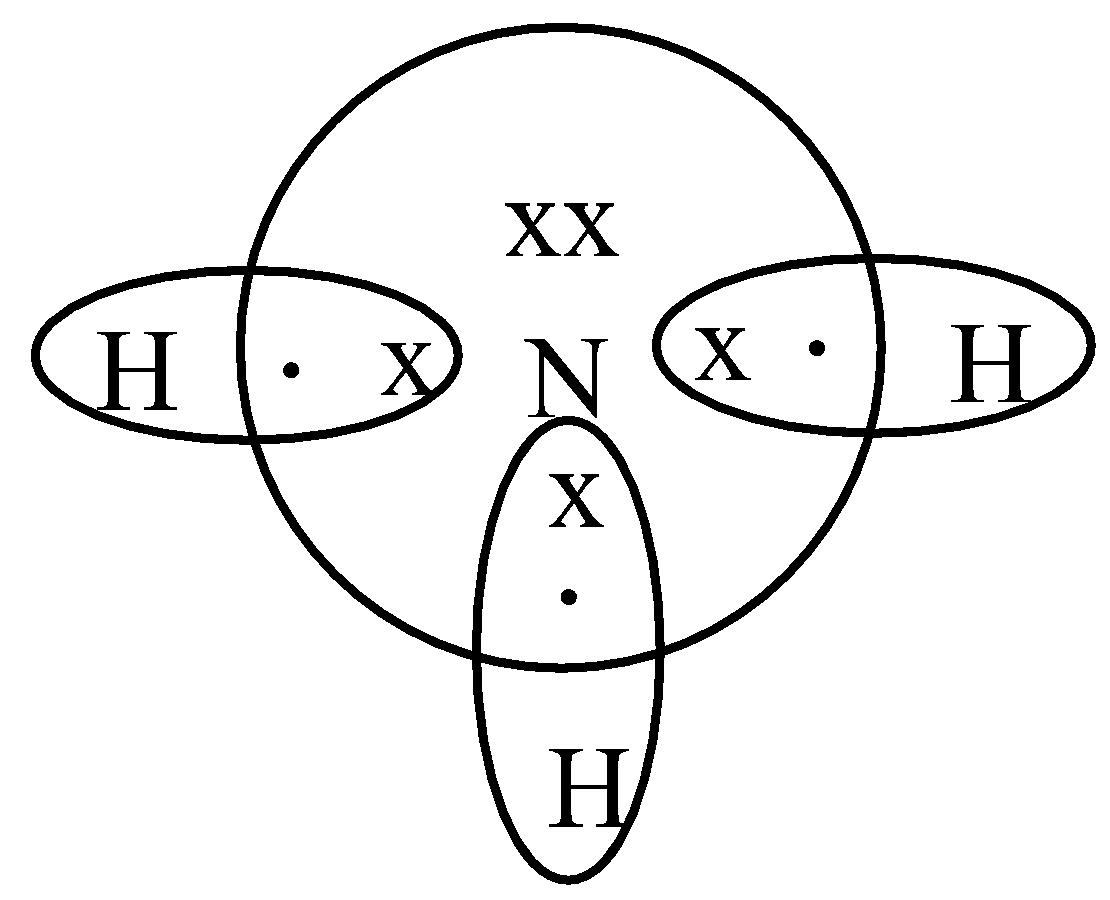
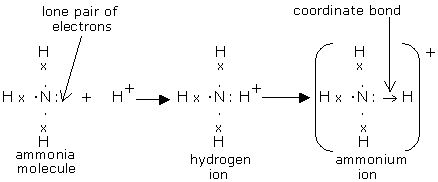
After counting the valence electrons, we have a total of 9 [5 from nitrogen + 4 (1 from each hydrogen)] = 9. and put a dot and a cross in each overlapping section (there should be three dots and three crosses in the whole diagram Actually the formula for an ammonia ion is NH4+ Due to dative bonding the 4th hydrogen loses an electron making it a ...
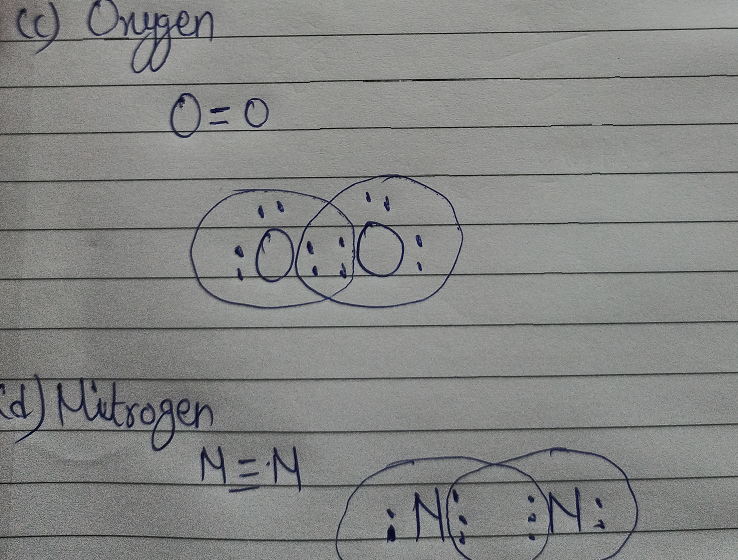
Draw an electron dot diagram for ammonia
NH 4 + lewis structure. You see, there are four hydrogen atoms around nitrogen atom. Therefore, nitrogen atom is the center atom. Also, there is a +1 charge on nitrogen atom. Steps of drawing lewis structure of NH 4 + There are several steps to draw the lewis structure of NH 4 +. But, These steps are explained in detail in this tutorial. Draw the dot and cross diagram with the outer shells overlapping. Then draw the shared electrons inside the overlapping section. Nitrogen gas occurs naturally as a diatomic molecule. The bond between the two nitrogen atoms is a triple bond. Again, draw the dot and cross diagram with the outer shells overlapping. Hi, im trying to find a enthalpy/concentration diagram for ammonia-water, but i' ve not find it on any of my currents books and on internet the image quality make them not readable, any ideas where to find one ?
Draw an electron dot diagram for ammonia. Hello do you guys know where i can get a xy diagram for the mixture ammonia and water? i cant seem to find a source which provides this diagram. Thank you for your help in advance In this how-to video, you will learn how to make the Lewis structure for Ammonia. The formula for Ammonia is NH3. Now, write down H, N, and H in a horizontal line. Place an H under the N. Place two dots in between the spaces found in the H's and the N. Also place two dots above the N. Since the valance electrons are balanced, draw a line between the two dots connecting the H to the N. Leave ... In addition to this, ammonia is considered corrosive as well as hazardous if stored in significantly larger quantities. The lewis structure that is also called an electron dot structure, is mainly a pictorial representation of the valence electrons present in an atom. The diagram is drawn using dots around the symbol of an atom, mostly in pairs. Hi guys. I'm a high school student researching some Quantum Electrodynamics for one of my school projects and I'm attempting to understand only the concepts for now. I was reading up on the Feynman Diagram describing the interaction between two electrons. It has two electrons being deflected, with a straight line through the middle representing a virtual photon being emitted from one of the electrons. My problem with this is that I have read everywhere that virtual particles must come in pairs. ...
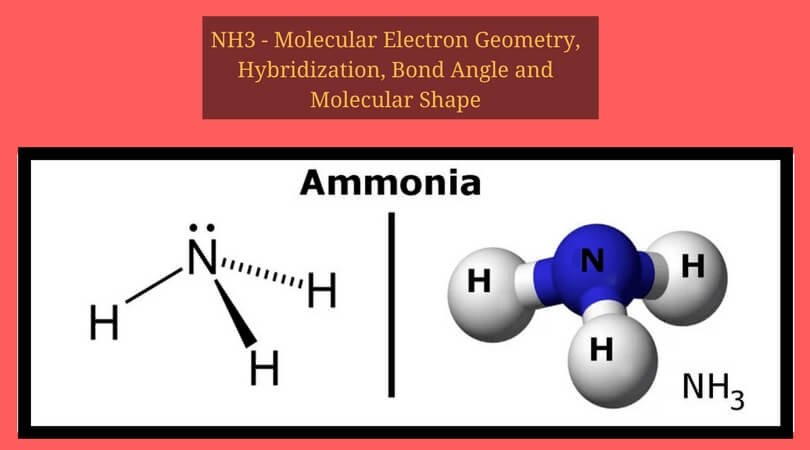
The Lewis dot structure of ammonia, NH3, reveals that it has one lone pair of electrons and three bonds (each to a hydrogen) around the central nitrogen atom. According to 13,568 results ... Draw the Lewis dot diagram of CO2, how many types of electrons are in the valence bond? The Lewis structure of ammonia, NH3, would be three hydrogen atoms bonded to a nitrogen atom in the middle, with a lone pair of electrons. Electron Dot Structure of NH3 by Jeff Bradbury - February 17, - Lewis Electron Dot Structure for ammonia molecule NH3. Reference the "How to Draw a Lewis Dot Structure" for a Step by Step guide. See the following Lewis dot structure diagrams for a few covalent compounds. Example 1. Ammonia, NH 3. Nitrogen is in Group V which means it has a total of five (5) valence electrons. There are three (3) hydrogens present, each with their own sole electron giving ... Nov 14, 2018 — Find an answer to your question draw the electron dot structure of ammonia molecule and show the formation of Ammonium Ion from meet level ...2 answers · 478 votes: plzzzzzzzzz mark my answer as brainliest
Question: 36. Draw the Lewis dot structure for ammonia (NH3). Indicate its shape, and specify whether it is a polar or non-polar molecule. 36. Draw the Lewis dot structure for ammonia (NH3). Indicate its shape, and specify whether it is a polar or non-polar molecule (5 pts.) 37. Describe the difference between an acid and a base (5 pts). The Lewis dot structure of ammonia, NH3, reveals that it has one lone pair of electrons and three bonds (each to a hydrogen) around the central nitrogen atom. ... Draw a Lewis dot diagram for NF2H and use the oxidation state method of electron bookkeeping to determine how many electrons each atom should be assigned. In the box below, draw the electron-dot (Lewis) structure of carbon di0>äde. In the box below, draw a Lewis electron-dot diagram for a molecule of phosphorus trichloride, PC13 (c) ammonia 60) 61) 62) 65) In the box provided, draw a Lewis electron-dot diagram for a molecule of chlorine, Ch. in terms of electrons, why the bonding in NaCl is Ionic. Ammonia (NH 3) is a commonly tested Lewis structure due to it's widespread use in agriculture as a fertilizer.It also is a good example of a molecule with a trigonal prymidal molecular geometry. There are 8 valence electrons available for the Lewis structure for NH 3.. Video: Drawing the Lewis Structure for NH 3
OK I am having major fundamental issues picturing carrier behaviour in p-n junctions. I have 2 good examples of where my logic doesn't make sense: 1) In a p-n junction with a small forward bias with less than the band gap energy, some majority carriers are injected in their respective side, and the carrier diffuses across to recombine when it is the minority carrier. Now if i track this path along an Energy vs. distance axis, at some point I will find an energy change corresponding to the band ...
What is the Lewis dot structure for ammonia? In the lewis structure of ammonia (NH3), there are three N-H bonds and one lone pair on nitrogen atom. Lewis structure of NH3 can be drawn by starting from valence electrons of nitrogen and hydrogen atoms in several steps. Can you draw the electron dot structure of […]
I'm currently doing my biggest project to date in which I have to "design and build a dynamic light creating object". Unfortunately I have very limited knowledge of electronics so I thought I'd make it easier for myself by buying the lighting part pre-made and reverse engineering it in order to build my own version. Could anyone tell me what each of the components are/ their purpose so that I could draw a circuit diagram for my logbook and work from there to recreate something similar? Thanks in...
This is the structure of ammonia or \[N{{H}_{3}}\].-In ammonium ion, the lone pair on nitrogen atoms of ammonia has the ability to fully share its pair with hydrogen ions, thus forming a coordination bond with nitrogen. Nitrogen will attain a positive charge on it. Thus it becomes an ammonium ion. The electron dot diagram is shown below.
In this video I draw the Dot and Cross Diagram for NH3 (Ammonia). Dot-and-cross diagrams are one way to represent covalent bonds in molecules. They are sim...
How to draw a dot and cross diagram for ammonia How to draw double bonds Step 1 Step 3 Step 2 Step 4 Once you have mastered electron configuration diagrams, you can adapt them to show structure and bonding in covalent and ionic compounds. These structural diagrams depict only the outer, or valence, shell electrons and are known as dot and cross ...
Alternatively a dot method can be used to draw the lewis structure of NH3. Calculate the total valence electrons in NH3 molecule. N=5,H=1x3=3 Total=8 Put Nitrogen in the center and three hydrogen atoms on the sides. We're going to do the Lewis structure for NH3: ammonia or Nitrogen trihydride. On the periodic table, Nitrogen is in group 5 or 15 ...
The Lewis structure of ammonia, #NH_3#, would be three hydrogen atoms bonded to a nitrogen atom in the middle, with a lone pair of electrons on top of the atom.This is the reason why ammonia acts as a Lewis base, as it can donate those electrons.
Lewis Structure Examples. The Lewis electron dot structures of a few molecules are illustrated in this subsection. 1. Lewis Structure of CO2. The central atom of this molecule is carbon. Oxygen contains 6 valence electrons which form 2 lone pairs. Since it is bonded to only one carbon atom, it must form a double bond.
Ammonia donates its lone pair to proton forming ammonium ion. Solve any question of Chemical Bonding and Molecular Structure with:-Patterns of problems > Was this answer helpful? 0. 0. Similar questions. Draw the electron dot structure of Nitrogen molecule [N = 7] Medium. View solution > Draw the electron dot structure for propanal.
A step-by-step explanation of how to draw the NH3 Lewis Dot Structure (Ammonia).For the NH3 structure use the periodic table to find the total number of vale...
10+ Lewis Dot Structure For Nh3. Watch the video and see if you missed any steps or information. Remember, too, that hydrogen only needs two valence electrons to have a full outer shell. Unit 4 Review Objectives 11-19 Flashcards | Quizlet from o.quizlet.com 4+3+5+2=14 ch3nh2 then draw the skeleton structure…
Our chemistry teacher said that he would prefer us not to simply take it from google images, and I was wondering if there was any alternative to handwriting them and taking a picture.
Which of the following is true regarding the Lewis diagram for ammoni a? (2 points) a. Ammonia contains three covalent bonds, one to each hydrogen atom, and the nitrogen atom has no lone pairs of electrons. b. Question: Draw the Lewis electron dot diagram for ammonia (NH3) on a sheet of scrap paper. You may consult the periodic table on Zoom to ...
36. Draw the Lewis dot structure for ammonia (NH3). Indicate its shape, and specify whether it is a polar or non-polar molecule (5 pts.) 37. Describe the difference between an acid and a base (5 pts). 38. Balance the following chemical equation by inspection (7 pts.).
NH 3 lewis structure. In the lewis structure of NH 3, there are three N-H bonds and one lone pair on nitrogen atom.There are no lone pairs on hydrogen atoms which cannot keep more than two electrons. Steps of drawing lewis structure of NH 3. You have to follow several steps to draw the lewis structure of NH 3.But, because ammonia is a simple molecule, these steps are not complex and do not ...
Hi, im trying to find a enthalpy/concentration diagram for ammonia-water, but i' ve not find it on any of my currents books and on internet the image quality make them not readable, any ideas where to find one ?
Draw the dot and cross diagram with the outer shells overlapping. Then draw the shared electrons inside the overlapping section. Nitrogen gas occurs naturally as a diatomic molecule. The bond between the two nitrogen atoms is a triple bond. Again, draw the dot and cross diagram with the outer shells overlapping.
NH 4 + lewis structure. You see, there are four hydrogen atoms around nitrogen atom. Therefore, nitrogen atom is the center atom. Also, there is a +1 charge on nitrogen atom. Steps of drawing lewis structure of NH 4 + There are several steps to draw the lewis structure of NH 4 +. But, These steps are explained in detail in this tutorial.










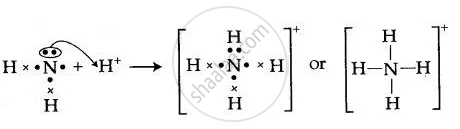


![Draw an electron dot diagram to show the formation of ammonium ion [Atomic number: N = 7 and H = 1 ].](https://i.ytimg.com/vi/t_Z8qSTAfr0/mqdefault.jpg)





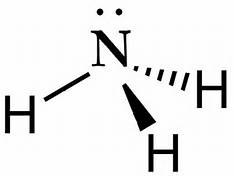
![Draw the electron dot structure of Ammonium ion [N = 7, H = 1]](https://d1hj4to4g9ba46.cloudfront.net/questions/1890011_1909650_ans_86e4b88529e541639e84039753e5b955.png)







0 Response to "40 draw an electron dot diagram for ammonia"
Post a Comment