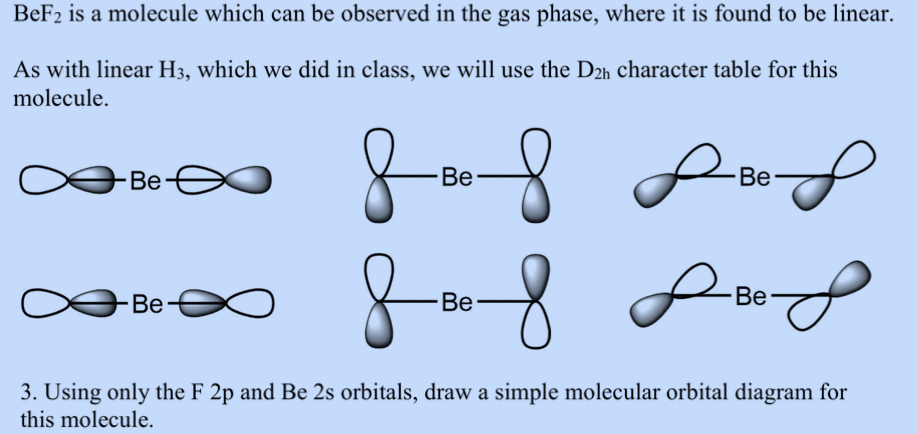
37 bef2 molecular orbital diagram
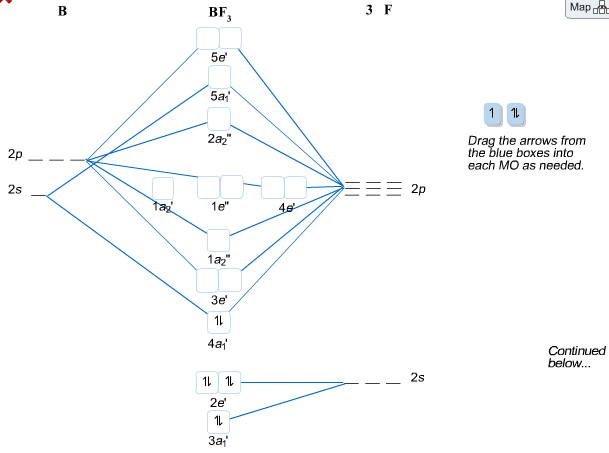
Beryllium fluoride is an odorless white solid. Denser than water. (USCG, 1999) CAMEO Chemicals. Beryllium difluoride is the fluoride salt of beryllium (+2 oxidation state). In the solid state it exists as a glass, with four-coordinate Be (2+) tetrahedral centres and two-coordinate fluoride centres. Bef2 Molecular Orbital Diagram When we apply valence-bond theory to polyatomic molecules, we must For the Be atom of BeF2, we write the orbital diagram for the formation of two sp hybrid. Create a molecular orbital diagram of the linear BeF2 molecule. For Be use a basis set that consists of the 2s, 2px, 2py, 2pz atomic orbitals.
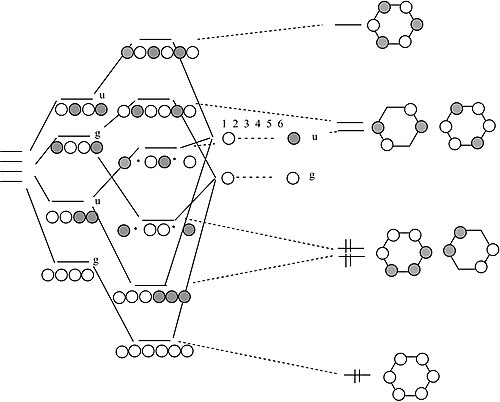
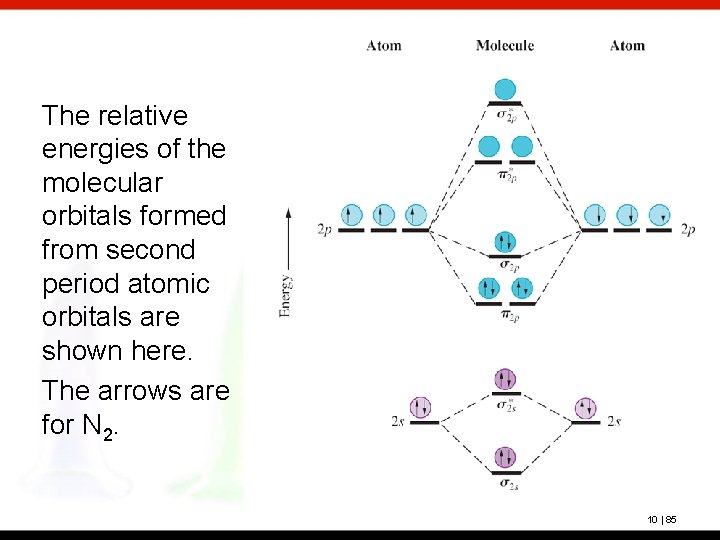
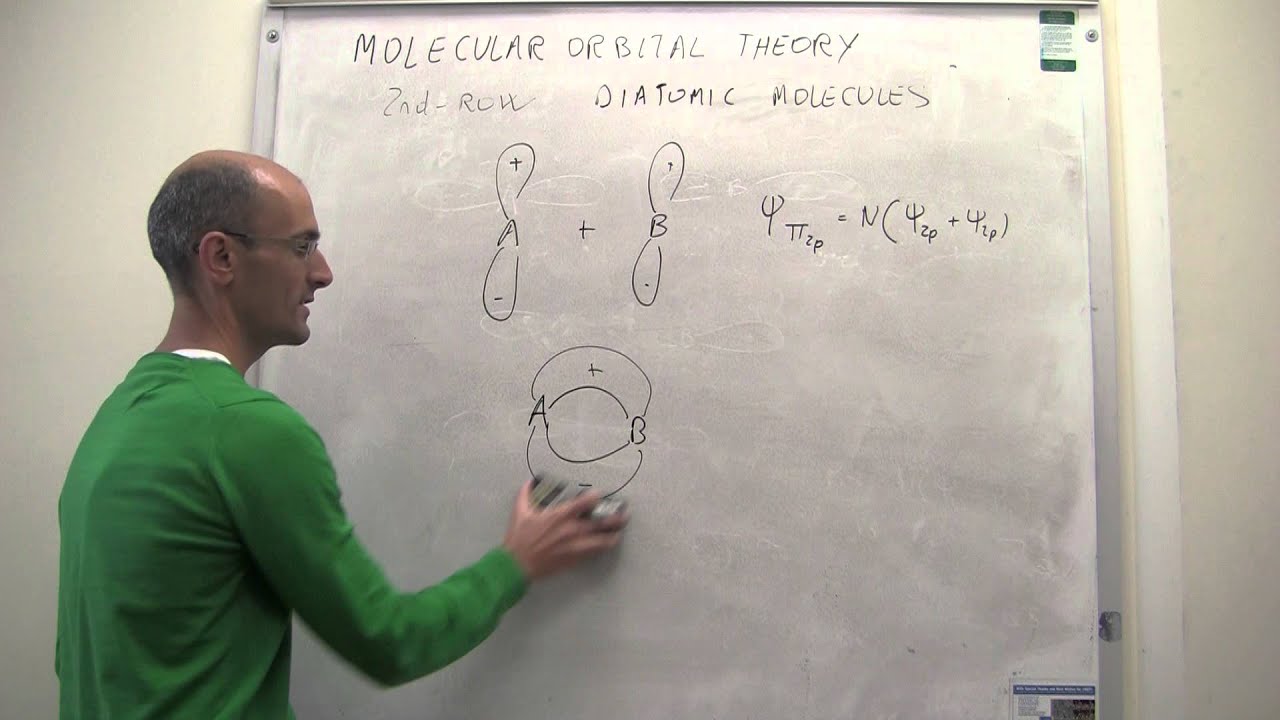
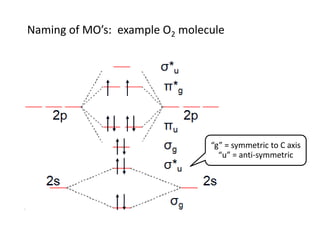
Draw the molecular orbital energy level diagram for each of the following species Be2+, Be2, and Be Indicate theirnumbers of unpaired electron and mention their magnetic diagramweb.netate their bond orders, and state which species is moststable% (1). Molecular orbitals of Li 2, Be 2, to F 2 The molecular orbital theory (MO) has been introduced ...

Bef2 molecular orbital diagram
BeF2 uses s and p orbitals on all three atoms, and is isoelectronic with CO2. The energy level. Be able to construct molecular orbital diagrams for homonuclear diatomic, heteronuclear diatomic, homonuclear triatomic, and heteronuclear triatomic molecules. Determine whether the following molecular orbitals are bonding or antibonding. ( c. 8 pts.) Nov 01, 2019 · RE: a thank you to entice the C-N molecular orbital diagram for the sigma bond in HCN? Label each and every molecular orbital with its call. Create a molecular orbital diagram of the linear BeF2 molecule. For Be use a basis set that consists of the 2s, 2px, 2py, 2pz atomic orbitals. Unlike in question 4. Create a molecular orbital diagram of the linear BeF2 molecule. For Be use a basis set that consists of the 2s, 2px, 2py, 2pz atomic orbitals. Unlike in question 4 here we will incorporate π bonding orbitals. The basis set for each F atom will be the py, px, and pz orbitals shown below. (Draw and label your orbitals as σb, π, σ* etc.)
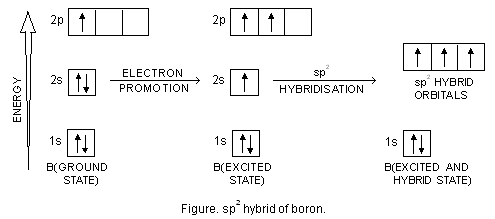
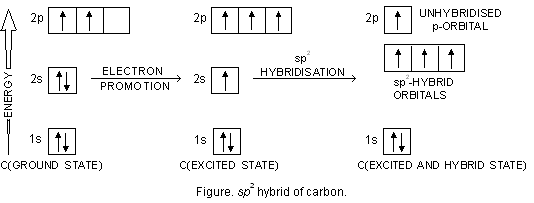
Bef2 molecular orbital diagram. BeF2 is a nonpolar molecule because of symmetrical geometry that causes the uniform distribution of charge in the molecule that leads to its net dipole moment zero. In the BeF2 Lewis dot structure, a total of 6 lone pairs and 2 bonded pairs are present. The molecular geometry of BeF2 is linear and its electron geometry is also linear in nature. In BeF 2, Be atom is sp hybridised i.e. two hybrid orbitals are directed along a straight line with a bond angle equal to 180°. Each sp hybrid orbital overlaps axially with 2p half-filled orbital of F atom to form sigma Be – F bonds. What hybridization is present in BeF2? These two sp hybridised orbitals get arranged in a linear shape. In the gas phase, BeF 2 forms linear monomeric molecules. Prepare a molecular orbital energy-level diagram for BeF 2, showing clearly which atomic orbitals are involved in bonding and which are nonbonding. Step-by-step solution 100% (5 ratings) for this solution Step 1 of 3 Step by step video for producing the MO diagram for BeF2. Captioned within the video but no audio.
Molecular Geometry: Molecular geometry is an area of focus in both inorganic and organic chemistry. In covalent chemistry, reaserachers focus on orbital hybridization and bond angles at central ... Create a molecular orbital diagram of the linear BeF2 molecule. For Be use a basis set that consists of the 2s, 2px, 2py, 2pz atomic orbitals. Unlike in question 4 here we will incorporate π bonding orbitals. The basis set for each F atom will be the py, px, and pz orbitals shown below. (Draw and label your orbitals as σb, π, σ* etc.) Nov 01, 2019 · RE: a thank you to entice the C-N molecular orbital diagram for the sigma bond in HCN? Label each and every molecular orbital with its call. Create a molecular orbital diagram of the linear BeF2 molecule. For Be use a basis set that consists of the 2s, 2px, 2py, 2pz atomic orbitals. Unlike in question 4. BeF2 uses s and p orbitals on all three atoms, and is isoelectronic with CO2. The energy level. Be able to construct molecular orbital diagrams for homonuclear diatomic, heteronuclear diatomic, homonuclear triatomic, and heteronuclear triatomic molecules. Determine whether the following molecular orbitals are bonding or antibonding. ( c. 8 pts.)














0 Response to "37 bef2 molecular orbital diagram"
Post a Comment