35 free energy reaction coordinate diagram
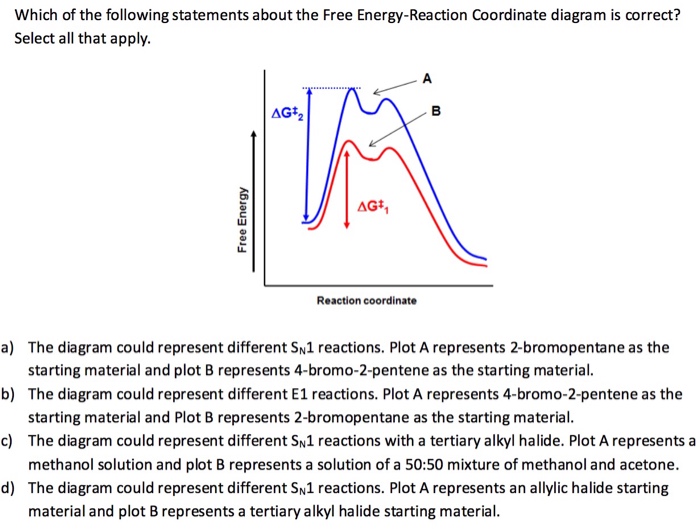
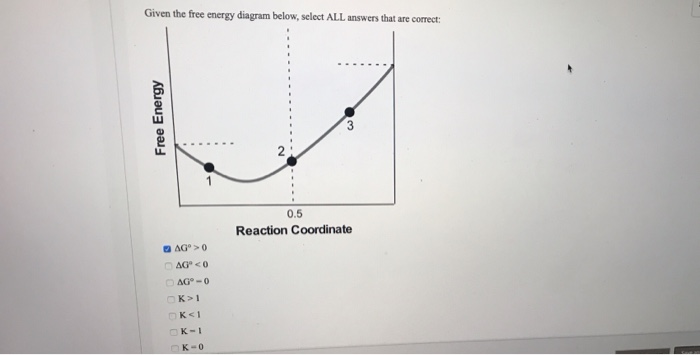
21) Which of the following statements about the Free Energy-Reaction Coordinate diagram is incorrect? A AG+ B AG+ Free Energy Reaction coordinate a) The diagram could represent different Sn1 reactions. Plot A represents the tertiary alkyl halide and plot B an allylic alkyl halide. b) The diagram could represent the addition of HCl to propene. A Reaction Coordinate Diagram for a One-Step Process (a) reactants (b) transition state (c) products (d) ΔG˚ Gibbs standard free energy change (e) ΔG≠ free energy of activation We have already encountered a useful tool that allows us to track energy changes along a complex reaction pathway - the tool was the reaction coordinate diagram
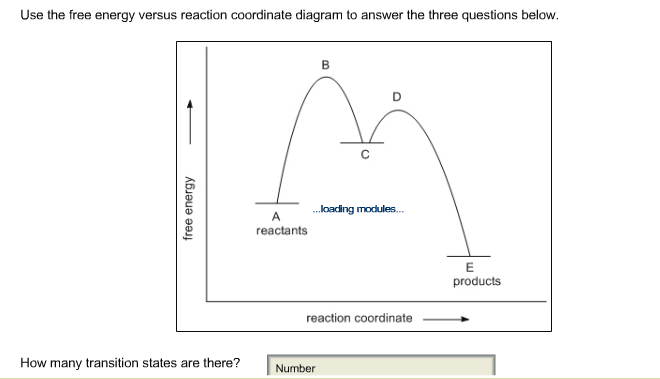
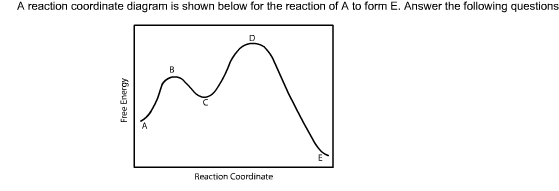
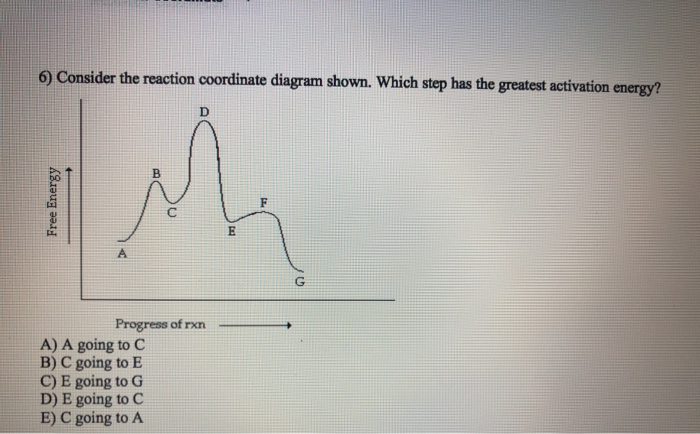
Figure 6:Reaction Coordinate Diagrams showing reactions with 0, 1 and 2 intermediates: The double-headed arrow shows the first, second and third step in each reaction coordinate diagram. In all three of these reactions the first step is the slow step because the activation energy from the reactants to the transition state is the highest.

Free energy reaction coordinate diagram
Reaction coordinate diagrams for exergonic and endergonic reactions. In the exergonic reaction, the reactants are at a higher free energy level than the products (reaction goes energetically downhill). In the endergonic reaction reaction, the reactants are at a lower free energy level than the products (reaction goes energetically uphill). This chemistry video tutorial focuses on potential energy diagrams for endothermic and exothermic reactions. It also shows the effect of a catalyst on the f... From Wikipedia, the free encyclopedia Diagram of a catalytic reaction, showing the energy niveau depending on the reaction coordinate. For a catalysed reaction, the activation energy is lower. In chemistry, a reaction coordinate is an abstract one-dimensional coordinate which represents progress along a reaction pathway.
Free energy reaction coordinate diagram. Catalysis is the process of increasing the rate of a chemical reaction by adding a substance Catalyzed reactions have a lower activation energy (rate-limiting free energy of activation) than the corresponding uncatalyzed reaction, resulting in a higher reaction . This effect can be illustrated with an energy profile diagram. ΔGrxn = ΔH rxn − T ΔSrxn So, the reaction is necessarily endothermic, and the reaction coordinate diagram and potential energy diagram look similar. The main difference is again, you'd be using ΔH rxn instead, and your y-axis would be the potential energy, NOT the Gibbs' free energy. Answer link reaction coordinate diagram shows that the energy of activation for the reverse reaction is lowered by the catalyst as well. Enzymatic catalysis The ability of enzymes to catalyze reactions depends on their ability to interact directly and specifically with The reaction free-energy diagram in Fig. summarizes these ideas. The first step, ion-ization of the alkyl halide to a carbocation, is the rate-limiting step and thus has the transition reaction coordinate Br Figure Reaction free-energy diagram for the S.
The reaction coordinate diagram for this reaction plots the changes in Gibbs free energy (ΔG) during the conversion of reac-tants (R) to TS to products (P; Figure 1B). It can be seen that P is of lower free energy than R, so this reaction is thermodynamically fa-vorable, that is, it proceeds in the direction of P with the release of Reaction coordinate diagram for the bimolecular nucleophilic substitution (S N 2) reaction between bromomethane and the hydroxide anion. The minimum kinetic energy required for a reaction to occur is called the activation energy and is denoted by E a or ΔG ‡. The transition state or activated complex shown on the diagram is the energy barrier that must be overcome … A Reaction Coordinate (Energy) Diagram Thermodynamic Quantities Gibbs standard free energy change there are many ENDOTHERMIC reactions such as photosynthesis that occur.! An Exothermic (Exergonic) Reaction! 2 C 8 H 18 + 25 O 2 → 16 CO 2 + 18 H 2 O! A reaction with ∆H°0 is endothermic. reaction coordinate Br Figure 9.11 Reaction free-energy diagram for the S N1–E1 solvolysis reaction of (CH 3) 3CBr with ethanol.The rate-limiting step,ionization of the alkyl halide (red curve),has the transition state of highest standard free energy.The
positions of atoms during a reaction. Reaction coordinate Energy Energy Diagrams 6 • Transition state ‡: – An unstable species of maximum energy formed during the course of a reaction. – A maximum on an energy diagram. • Activation Energy, ∆G‡: The difference in Gibbs free energy between reactants and a transition state. Gibbs free energy reaction coordinate profiles found in some textbooks. The energy diagram for a reaction model consisting of one enzyme, one substrate, and one product is depicted in many books where it is compared with that for the uncatalyzed reaction. The survey of several Biochemistry textbooks reveals a high diversity of profiles for the ... Free-energy diagrams, or reaction coordinate diagrams, are graphs showing the energy changes that occur during a chemical reaction. The reaction coordinate represented on the horizontal axis shows how far the reaction has progressed structurally. Energy/Reaction Coordinate! Diagrams! Thermodynamics, Kinetics ! Dr. Ron Rusay" A Reaction Coordinate (Energy) Diagram Thermodynamic Quantities Gibbs standard free energy change (ΔGo) Enthalphy (ΔHo): the heat given off or absorbed during a reaction Entropy (ΔSo): a measure of freedom of motion ΔGo = ΔHo - TΔSo ΔG,ΔH,ΔS, ΔE are state ...
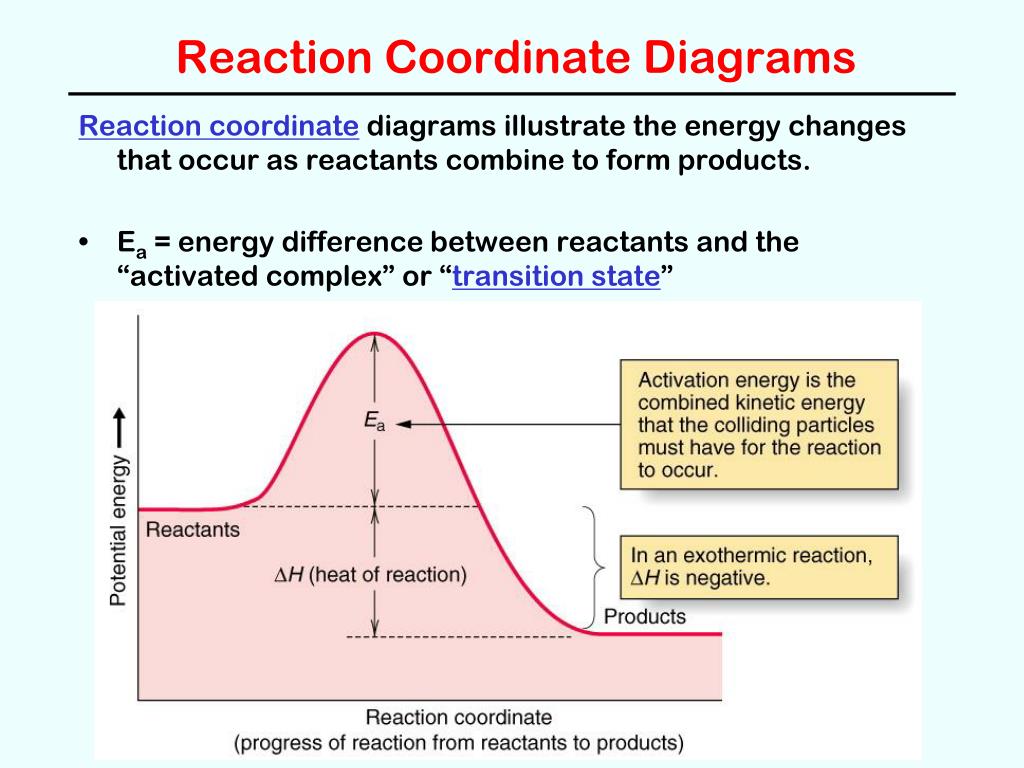
The diagram below is called a reaction coordinate diagram. It shows how the energy of the system changes during a chemical reaction. In this example, B is at a lower total energy than A. This is an exothermic reaction(heat is given off) and should be favorable from an energy standpoint. The energy difference between A and B is E in the diagram.
Figure 1: Reaction coordinate diagram for the bimolecular nucleophilic substitution (S N 2) reaction between bromomethane and the hydroxide anion. Transition state theory (TST) explains the reaction rates of elementary chemical reactions. The theory assumes a special type of chemical equilibrium (quasi-equilibrium) between reactants and activated transition state …
1) Free Energy of activation for B-> A is E Answer- E 2) Option …. View the full answer. Transcribed image text: What is the free energy of activation for reaction B A in the following reaction coordinate diagram? Reaction Coordinate Question 16 0.5 pts Which of the following carbocations is the least stable? CH3 1 CHỊCH IV.
coordinate diagrams", i.e. plots of energy (commonly free energy, ∆G) vs the "extent of the reaction". Reaction coordinate The "extent of the reaction" can, if necessary, be defined in terms of something quantitative, e.g. internuclear distances between reacting atoms, but for any but the simples processes this usually requires a switch from ...
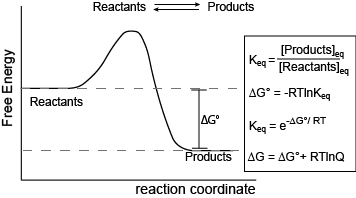
Reaction coordinate Transition state ΔG 1 ‡ ΔG 1 ‡' ΔG -1 ‡' ΔG -1 ‡ ΔG° G A G P Initial state Final state Fig. 4.2 A free energy (G) diagram for a simple reversible exothermic reaction A↔P(solid and broken lines). G A and G P represent the average free energies per mole for the reactant A and the product P, the initial and ...
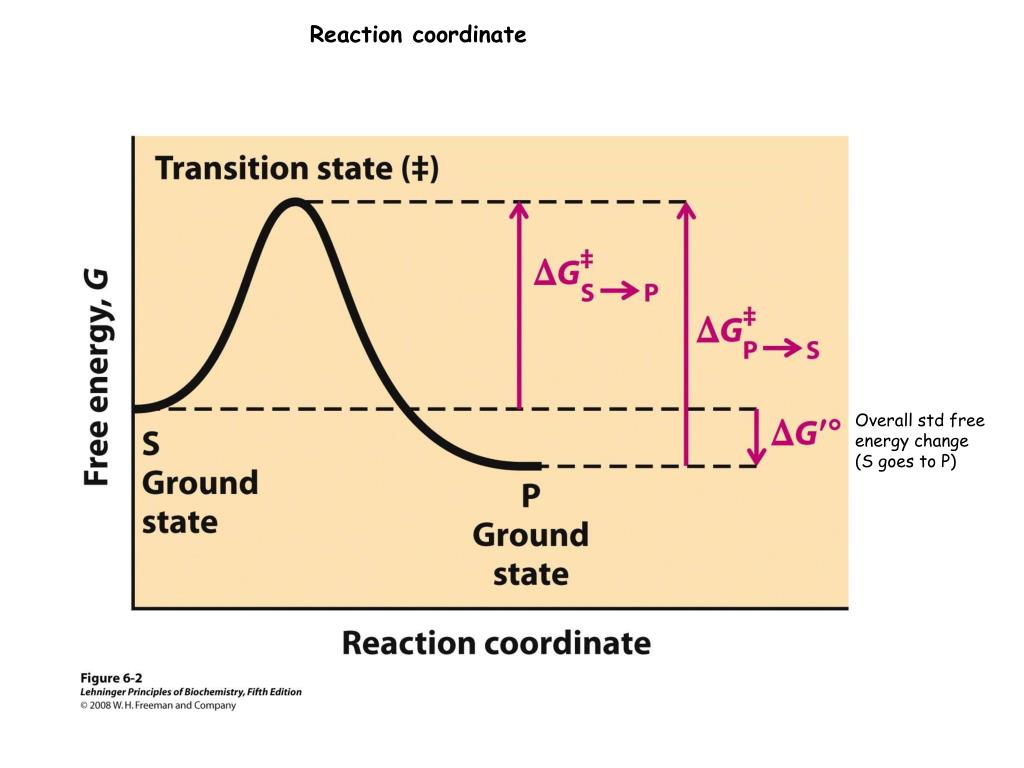
Biochemical standard free-energy change ΔG'° is the standard free-energy change at pH 7.0. Fig. 1 Reaction coordinate diagram for a chemical reaction Fig. 2 Reaction coordinate diagram comparing enzyme-catalyzed and uncatalyzed reactions.

Nuclear powerplant in Belgium Please mention me on Instagram: @Fredpaulussen or link to my website fredography.be Thank you!
1i. Draw an energy vs reaction coordinate diagram to illustrate a reaction in which the energy of the products is greater than the energy of the reactants. Label all quantities as per Fig. 1. See diagram (3) in sample exercise 14.10 on pg 595 of Brown and LeMay, 11th ed.
A reaction coordinate diagram is a graph that plots energy versus reaction progress. The amount of energy that needs to be added is called the activation energy, which is the point where the line ...
Energy Surfaces vs. Reaction Coordinate Diagrams Something that cannot be represented in the three dimensional energy surface below is the differentially populated vibrational modes of the molecules Reaction coordinate diagrams are used to simplify the situation, they represent a composite
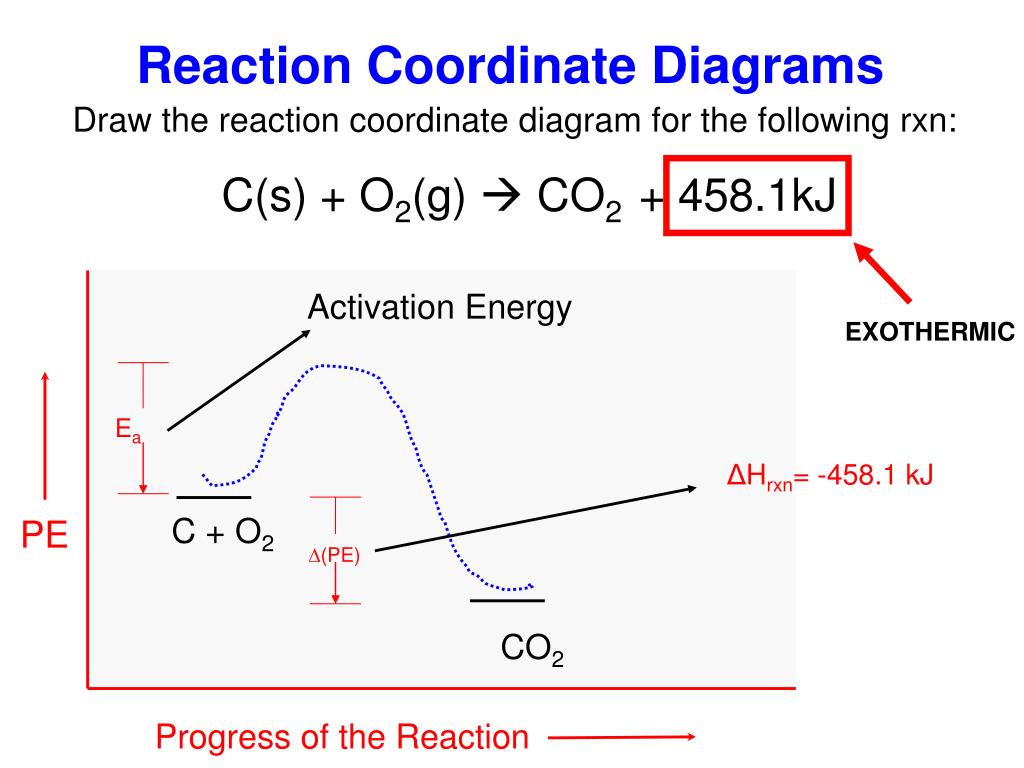
A general Reaction Coordinate Diagram relating the energy of a system to its geometry along one possible reaction pathway is given in the figure below. In the figure below, the Activation Energy, Ea is that critical minimum energy in a chemical reaction required by reactants to be converted into products. the quantities, Ea;
The fully filled in reaction coordinate diagram is displayed below. The arrow marked in the question represents the activation energy, which is the energy barrier that must be overcome in order for the reactants to form products. This reaction is also exothermic because the energy of the products is lower than that of the reactants.
For this quiz, you must be able to: Understand endothermic and exothermic reactions. Identify which points on sample reaction coordinate diagrams represent the activation energy and the change in ...
Typically, we envision reactions proceeding left to right along the reaction coordinate, so often, the activation energy is only noted for the forward reaction. The activation energy on the diagram below shows the barrier to be 102.6 kJ mol-1. Barriers are measured in energy per mole (typically kJ mol-1).
From Wikipedia, the free encyclopedia Diagram of a catalytic reaction, showing the energy niveau depending on the reaction coordinate. For a catalysed reaction, the activation energy is lower. In chemistry, a reaction coordinate is an abstract one-dimensional coordinate which represents progress along a reaction pathway.
This chemistry video tutorial focuses on potential energy diagrams for endothermic and exothermic reactions. It also shows the effect of a catalyst on the f...
Reaction coordinate diagrams for exergonic and endergonic reactions. In the exergonic reaction, the reactants are at a higher free energy level than the products (reaction goes energetically downhill). In the endergonic reaction reaction, the reactants are at a lower free energy level than the products (reaction goes energetically uphill).



















0 Response to "35 free energy reaction coordinate diagram"
Post a Comment