40 log c ph diagram
This information is most usefully expressed by means of a E-vs.-pH diagram, also known as a Pourbaix diagram. Figure \(\PageIndex{2}\) : Stability (Pourbaix) diagram for water. The two E° values shown at the left refer to "standard" conditions of unit H + activity (pH=0) and gas pressures of 1 atm. For the low-pH virus-inactivation process, we set the minimum pH range and longest processing time according to protein stability data. For comprehensive virus inactivation, it generally is recommended that the pH is ≤3.8, incubation time is ≥30 minutes, and the incubation temperature is ≥14 °C.
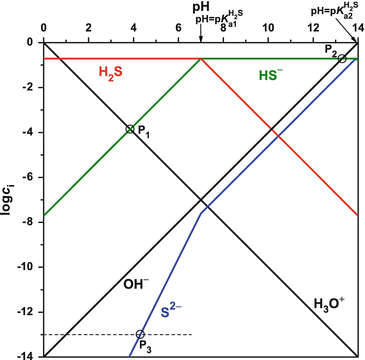
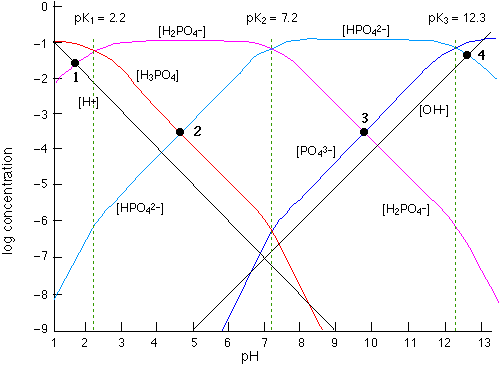
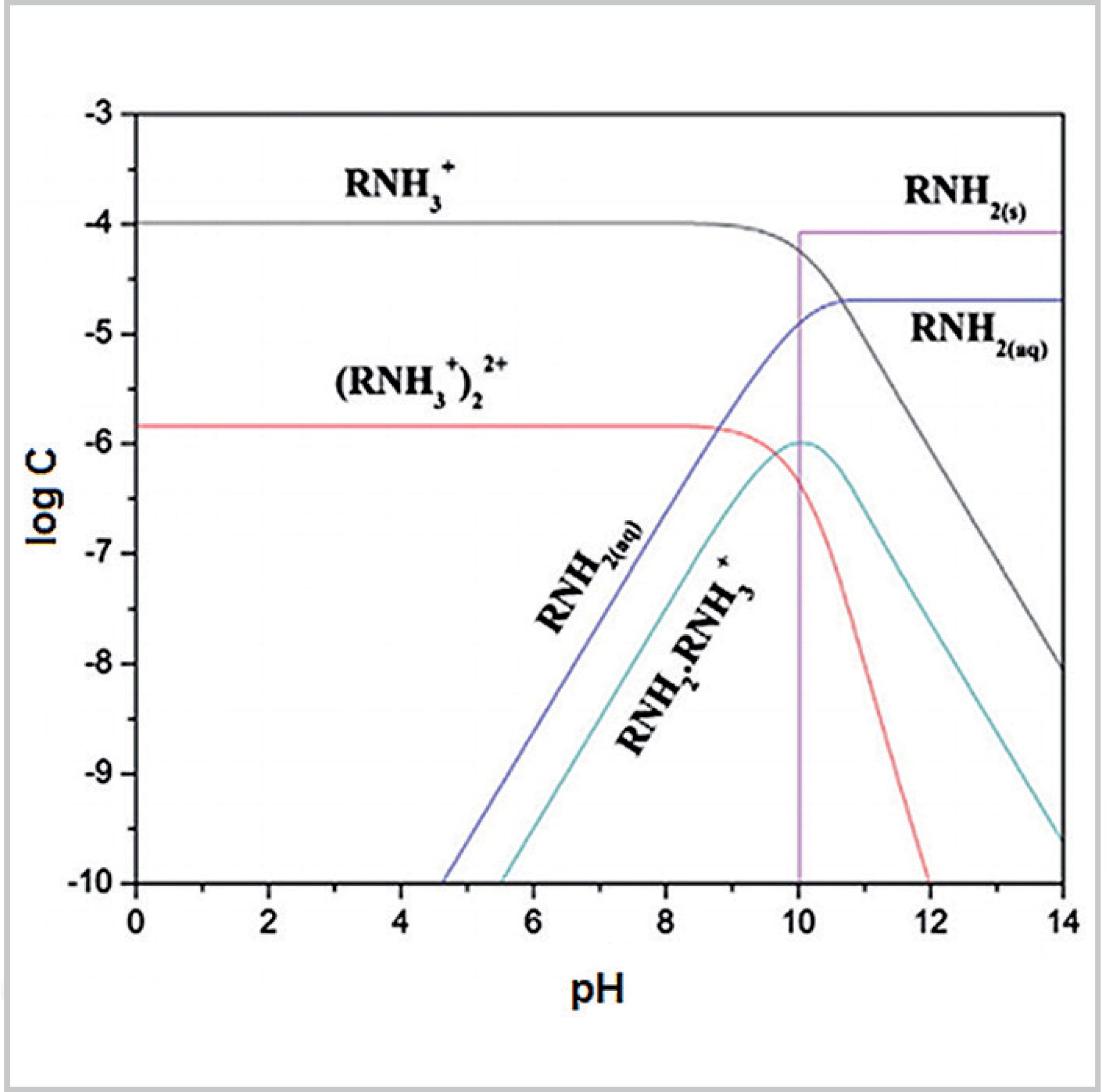
Log C-pH Diagrams. • The diagram should provide the concentrations of all the equilibrium species at all pH values. • The only equations used to make these ...

Log c ph diagram
Knowing the dependence of p H on [ H 3 O +], we can summarize as follows: If pH < 7, then the solution is acidic. If pH = 7, then the solution is neutral. If pH > 7, then the solution is basic. This is known as the p H scale. The range of value s from 0 to 14 that describes the acidity or basicity of a solution. Predict the approximate pH and the final composition after mixing together 0.090 moles of acetic acid and 0.040 moles of p‐nitrophenolate.. Solution. The ladder diagram in Figure 6.6.3 indicates that the reaction between acetic acid and p‐nitrophenolate is favorable.Because acetic acid is in excess, we assume the reaction of p‐nitrophenolate to p‐nitrophenol is complete. As described on the work slide, the area under a process curve on a p-V diagram is equal to the work performed by a gas during the process. On the right of the figure we have plotted the temperature versus the entropy of the gas. This plot is called a T-s diagram. Lines of constant pressure curve from the lower left to upper right on a T-s diagram.
Log c ph diagram. The word pH is acquired from "p," the scientific figure for negative logarithm, and "H," the chemical symbol for Hydrogen. pH is a unit of measure that expresses the level of acidity or alkalinity of a suspension. It is graded on a range of 0 to 14. pH = -log[H+] Acid-base without math: how to understand and use log-C vs pH diagrams. In mathematics, the logarithm is the inverse function to exponentiation.That means the logarithm of a given number x is the exponent to which another fixed number, the base b, must be raised, to produce that number x.In the simplest case, the logarithm counts the number of occurrences of the same factor in repeated multiplication; e.g. since 1000 = 10 × 10 × 10 = 10 3, the "logarithm base 10 ... Introduction to log C-pH diagram : A weak monoprotic acid example i. Draw [H+] line. -log [H+] = pH ... LogC-pH diagram for a weak acid and weak base.
Pourbaix diagrams are essentially electrochemical phase diagrams, which plot regions of thermodynamic stability for redox-active substances.As in other kinds of phase diagrams, the lines represent conditions under which two phases coexist in equilibrium. The shaded area in the water Pourbaix diagram represents the conditions of potential and pH where liquid water is stable relative to hydrogen ... 41 Harley Rear Wheel Assembly Diagram; 43 Log C Ph Diagram; 37 Millivolt Gas Valve Wiring Diagram; 39 2004 Monte Carlo Radio Wiring Diagram; 43 Where Is The Clitorus Diagram; 39 Remington Airmaster 77 Parts Diagram; 38 What Is A Stem And Leaf Diagram; 38 1999 Toyota Corolla Belt Diagram; 40 Frigidaire Freezer Parts Diagram; 43 3 Phase Motor ... The exact relationship depends on the activity of the hydrogen ion: (2) p H = − log 10. . a H +. Consequently, activities for hydrogen-ion in a given solution can be determined through simple pH measurements, and the activity coefficient ( γ) can be evaluated using the relationship. (3) a = γ C. Systematic Solution to Buffer Problems. Example 6.8.1 Exercise 6.8.1 Representing Buffer Solutions with Ladder Diagrams; Preparing a Buffer; Adding as little as 0.1 mL of concentrated HCl to a liter of H 2 O shifts the pH from 7.0 to 3.0. Adding the same amount of HCl to a liter of a solution that 0.1 M in acetic acid and 0.1 M in sodium acetate, however, results in a negligible change in pH.
Avoid math altogether and make a log-C vs pH plot. This is not only simple to do (all you need is a scrap of paper and a straightedge), but it will give you far more insight into what's going on, especially in polyprotic systems. All explained in Section 3 of the next lesson. The term enzyme specificity means that most enzymes: a) Only work at body temperature of 37 deg C. b) Only work at a particular pH value. c) Only catalyze one particular reaction. d) Are usually ... Lymph Node Anatomy. Lymph nodes range in size from 1-2 centimeters and are sometimes found alone, or in groups. These bean-shaped structures are composed of four main layers - the capsule ... It is clear that the pH at which the above buffers operate varies as the ionization constants vary, and the above diagram demonstrates the logic behind the rules in section 17.2.4.1 for and choosing a buffer, that is, you pick an acid with a K value near the pH (or pOH) and adjust the conjugate pair until it reaches that value.
pH = -log 10 (H +) The term is used to indicate basicity or acidity of a solution on a scale of 0 to 14, with pH 7 being neutral. As the concentration of H + ions in solution increases, acidity increases and pH gets lower, below 7 (see Figure 1). When pH is above 7, the solution is basic. ... Example of a simple conceptual model diagram for Low pH.
HACCP Food Safety Checklist. This HACCP Food Safety template helps to record potential food safety hazards which can be biological, chemical, or physical. Use this checklist to evaluate the CCPs, critical limits for each control measure, and frequency of the CCPs. Identify the corrective actions to be used and verify the activities performed.
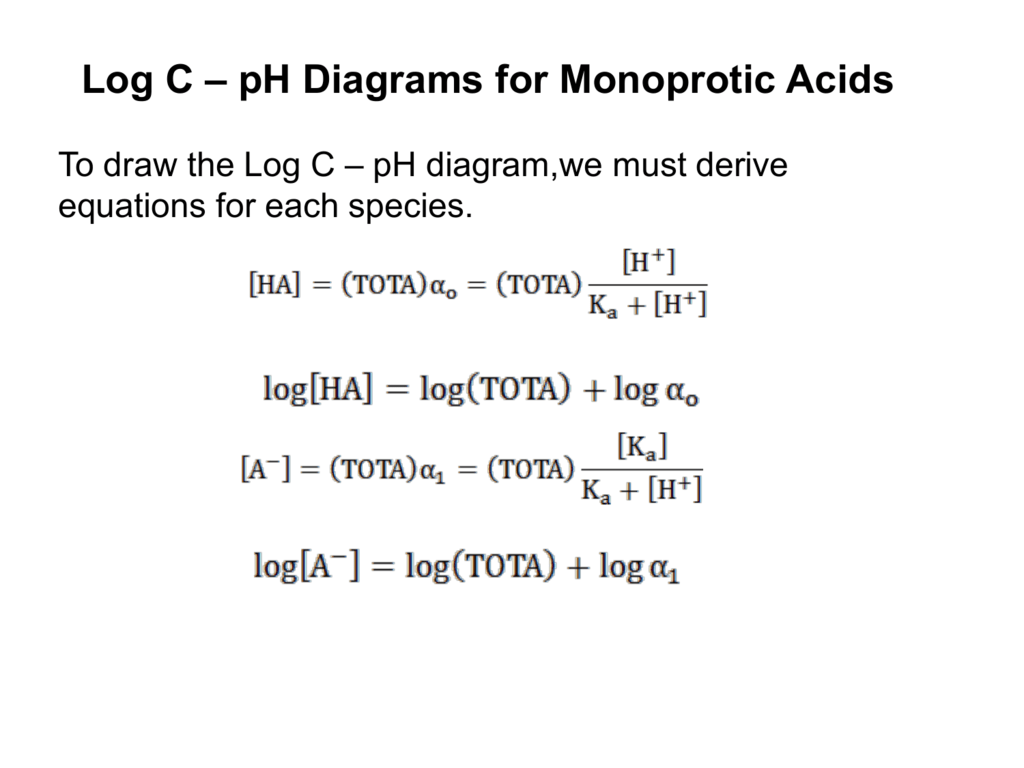
Log C – pH Diagrams for Monoprotic Acids To draw the Log C – pH diagram,we must derive equations for each species. Log C-pH Diagrams • The diagram should ...
The log phase, short for the logarithmic or exponential growth phase, is when bacterial cells actively divide by binary fission and exponentially increase in number after each generation time ...
The pH meter. The pH scale is a familiar concept for students who study science. The pH value of a solution reflects the relative concentration of hydrogen ions (H+) or protons to the concentration of hydroxide ions (OH-) in a solution. Solutions with a pH value less than 7 are acidic and those with a value greater than 7 are basic, or alkaline.
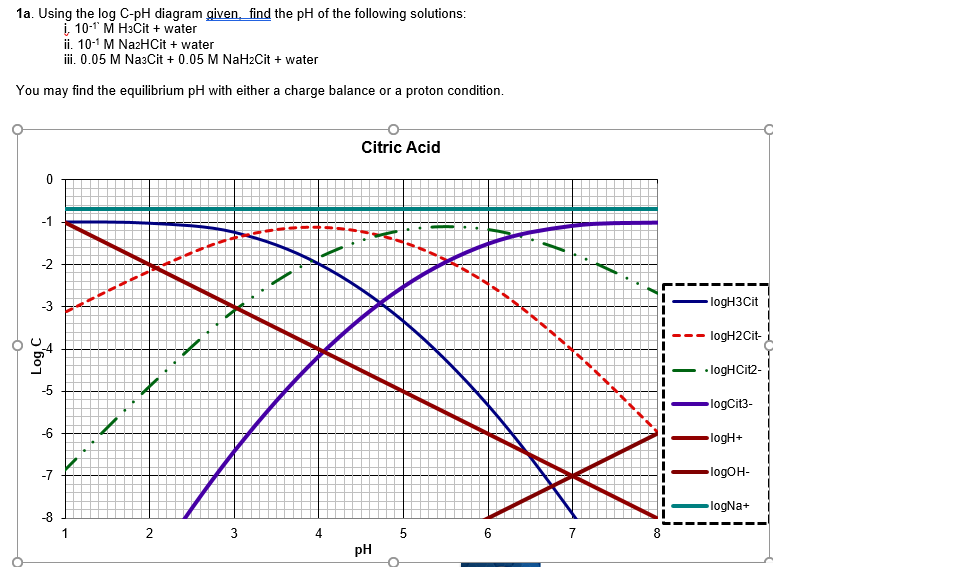
Prepare a complete Log C vs pH diagram for a system containing pure water and an excess of α-aluminum hydroxide (25ºC and I=0). (2 points).6 pages
These equations contain the terms of (∂E/∂pH) j and (∂E/∂log j) pH, which can be quantitatively addressed by looking at the E‐pH and the E‐log(j) plots, respectively. The slope of the E‐pH diagram in Figure 1 b corresponded to the (∂E/∂pH) j, determined to be −60 mV pH −1 in a wide pH range of 2-13
Aug 15, 2020 — The log-C vs. pH diagram is constructed as s superposition of plots for each conjugate pair at its respective pKa. Note especially that the pH ...pH of an acid in pure water · pH of a solution of the... · Putting it all together
2 Log C-pH Diagrams The diagram should provide the concentrations of all the equilibrium species at all ph values. The only equations used to make these ...
As described on the work slide, the area under a process curve on a p-V diagram is equal to the work performed by a gas during the process. On the right of the figure we have plotted the temperature versus the entropy of the gas. This plot is called a T-s diagram. Lines of constant pressure curve from the lower left to upper right on a T-s diagram.
Predict the approximate pH and the final composition after mixing together 0.090 moles of acetic acid and 0.040 moles of p‐nitrophenolate.. Solution. The ladder diagram in Figure 6.6.3 indicates that the reaction between acetic acid and p‐nitrophenolate is favorable.Because acetic acid is in excess, we assume the reaction of p‐nitrophenolate to p‐nitrophenol is complete.
Knowing the dependence of p H on [ H 3 O +], we can summarize as follows: If pH < 7, then the solution is acidic. If pH = 7, then the solution is neutral. If pH > 7, then the solution is basic. This is known as the p H scale. The range of value s from 0 to 14 that describes the acidity or basicity of a solution.

















0 Response to "40 log c ph diagram"
Post a Comment