39 electron dot diagram for h2o
H2O2 Lewis Structure, Molecular Geometry, Hybridization, and Polarity. H2O2 is a chemical compound with the IUPAC name Hydrogen Peroxide. It is the simplest peroxide compound, i.e., a molecule containing an Oxygen-Oxygen single bond. It is a pale blue liquid in its standard state and slowly reacts with sunlight and decomposes into water and oxygen. H2O's Lewis Dot Structure gives it many unique properties mostly due to the two lone pairs on the central oxygen atom. This increases electron-electron repulsion and therefore creates a bent structure as opposed to CO2's linear structure.This "bent" molecular structure gives it many unique properties such as being polar.One of the most fascinating phenomena is the idea of "hydrogen bonding ...
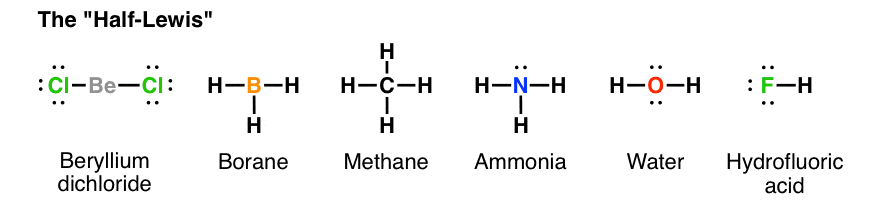
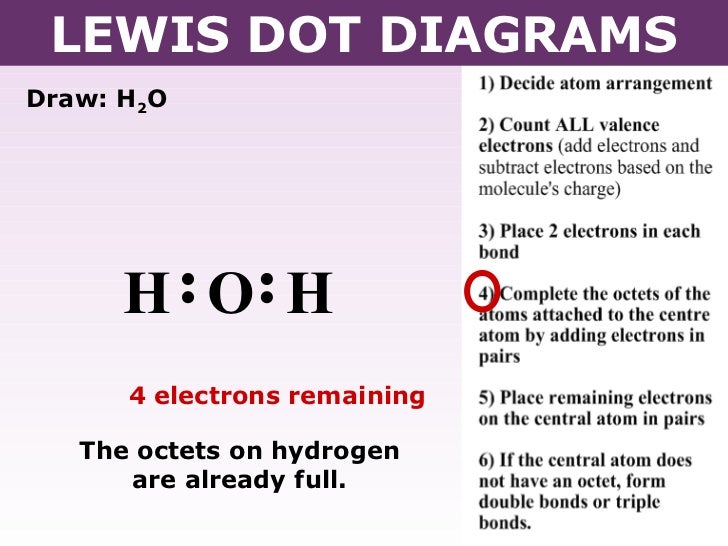
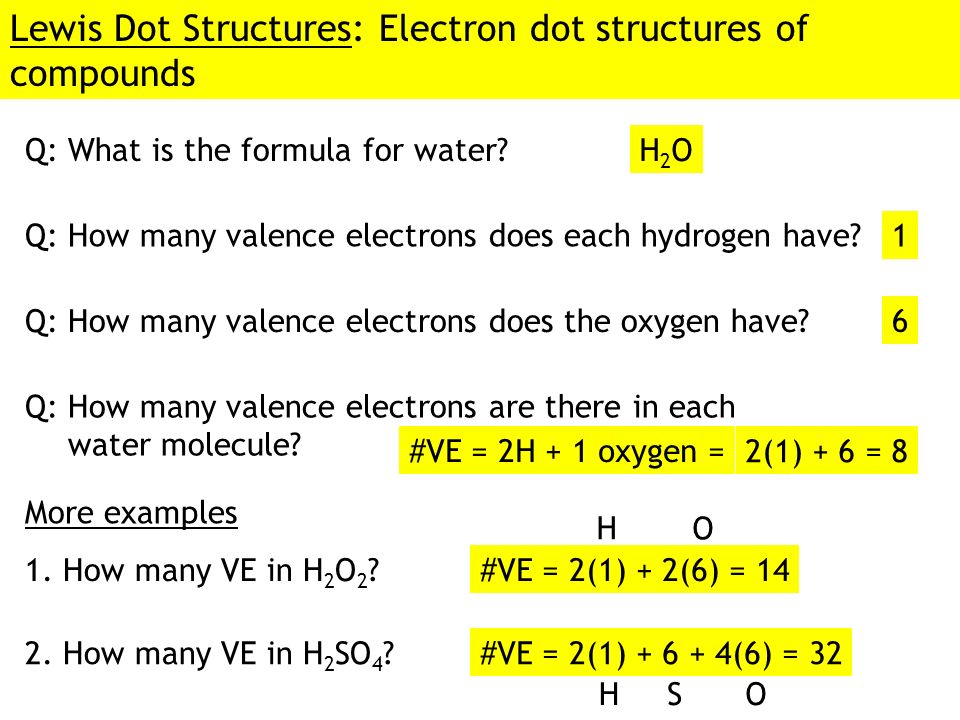
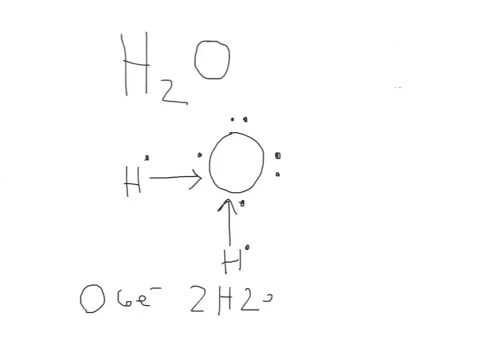
1 Answer. Ernest Z. Jul 15, 2014. You can find a procedure for drawing Lewis structures at this location. For H₂O, O must be the central atom. The skeleton structure is H-O-H. O has 6 valence electrons, and each H has one. You must arrange 8 electrons in pairs so that O has 8 and each H has two electrons in its valence shell.
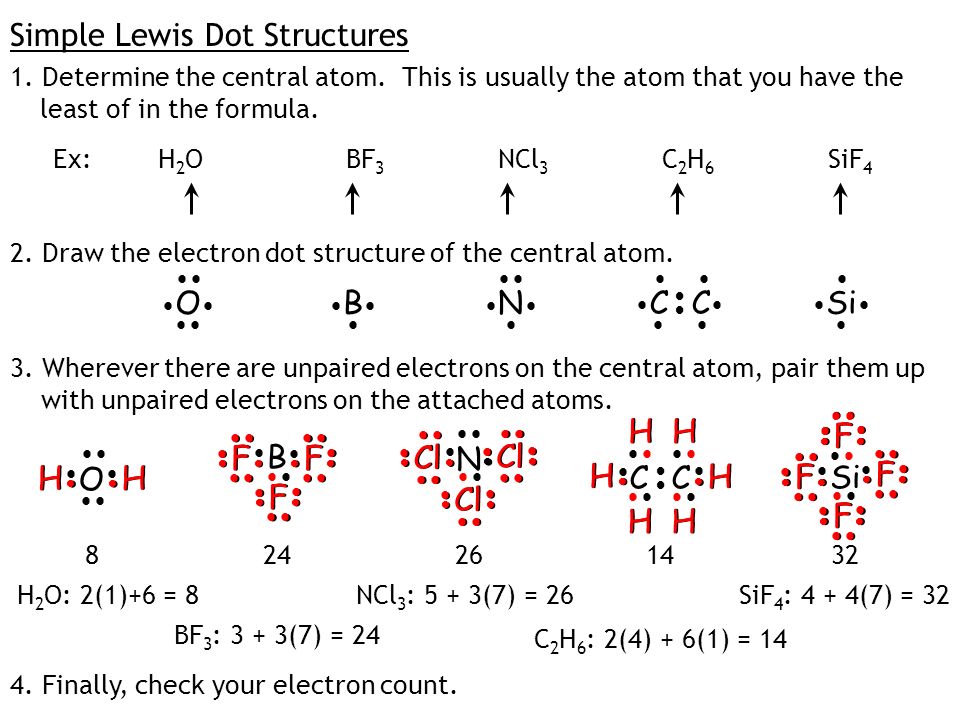
Electron dot diagram for h2o
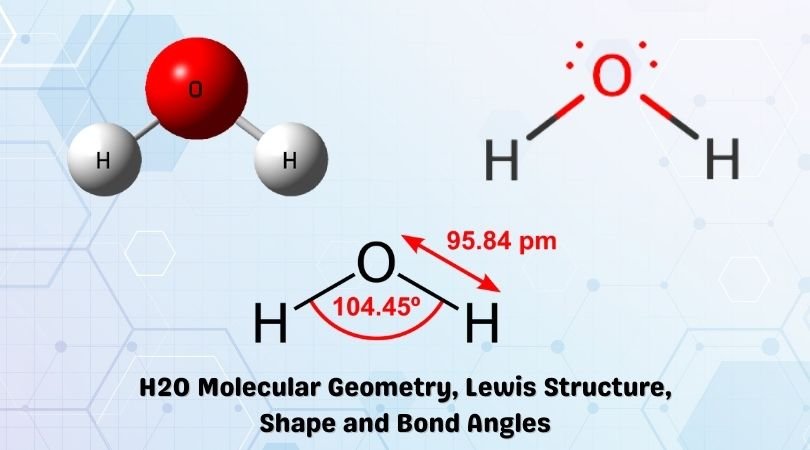
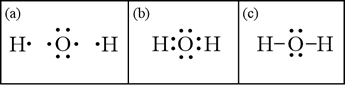
The Lewis dot structure for water shows the electron from hydrogen and an electron from oxygen being shared in a covalent bond. In order to determine the molecular geometry for H2O observe the Lewis structure of the same. I quickly take you through how to draw the Lewis Structure of water H2O. COVID-19 is an emerging rapidly evolving situation. Therefore, corresponding to the question, we can that the correct Lewis dot structure of water molecules is number 3. So, Option C is the correct answer. Note: (1) Lewis structures also known as Lewis dot structures or electron dot structures are diagrams that represent the valence electrons of an atom within a molecule. The molecular geometry of any molecule depends on its Lewis structure, the arrangement of atoms and its electrons. In H2O molecule, the Oxygen atom forms two single sigma bonds with Hydrogen atoms. Although these two Hydrogen atoms are arranged symmetrically in the plane, the two lone pairs of electrons on the Oxygen atom push these atoms.
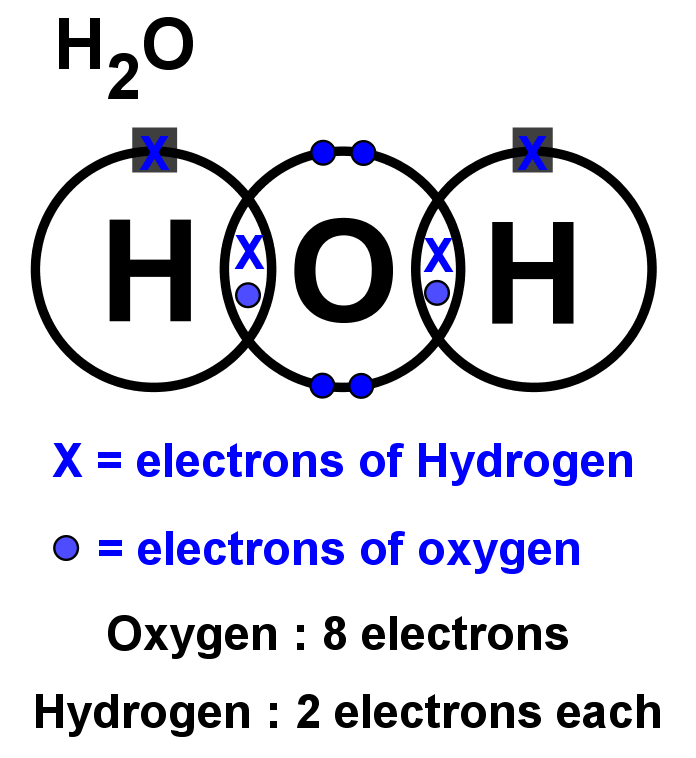
Electron dot diagram for h2o. Lewis structure diagram showing lone pairs and bonding pairs of electrons in a molecule or an ion Lewis symbol symbol for an element or monatomic ion that uses a dot to represent each valence electron in the element or ion lone pair two (a pair of) valence electrons that are not used to form a covalent bond octet rule In this manner, what is the electron dot structure of h2o? Drawing the Lewis Structure for H 2 O Another straight forward Lewis structure. You have a total of 8 valence electrons available to fill the octets of Oxygen and Hydrogen. Remember that Hydrogen only needs two electrons to have a full outer shell. H 2 O Lewis Structure Video. Video Transcript: Here, we're going to do a dot structure for water, H2O. Let's write that down: H2O. What we want to find out first is how many valence electrons does water have. I'm counting all the outer shell electrons. I'll need my periodic table. Correct option is. C. Oxygen atom has 6 valence electrons , out of which 2 are involved in bonding with the 2 hydrogen atoms. Therefore water molecule has 2 bonded pair of electrons and 2 lone pair (non bonded) pair of electrons and the correct electronic structure is (C).
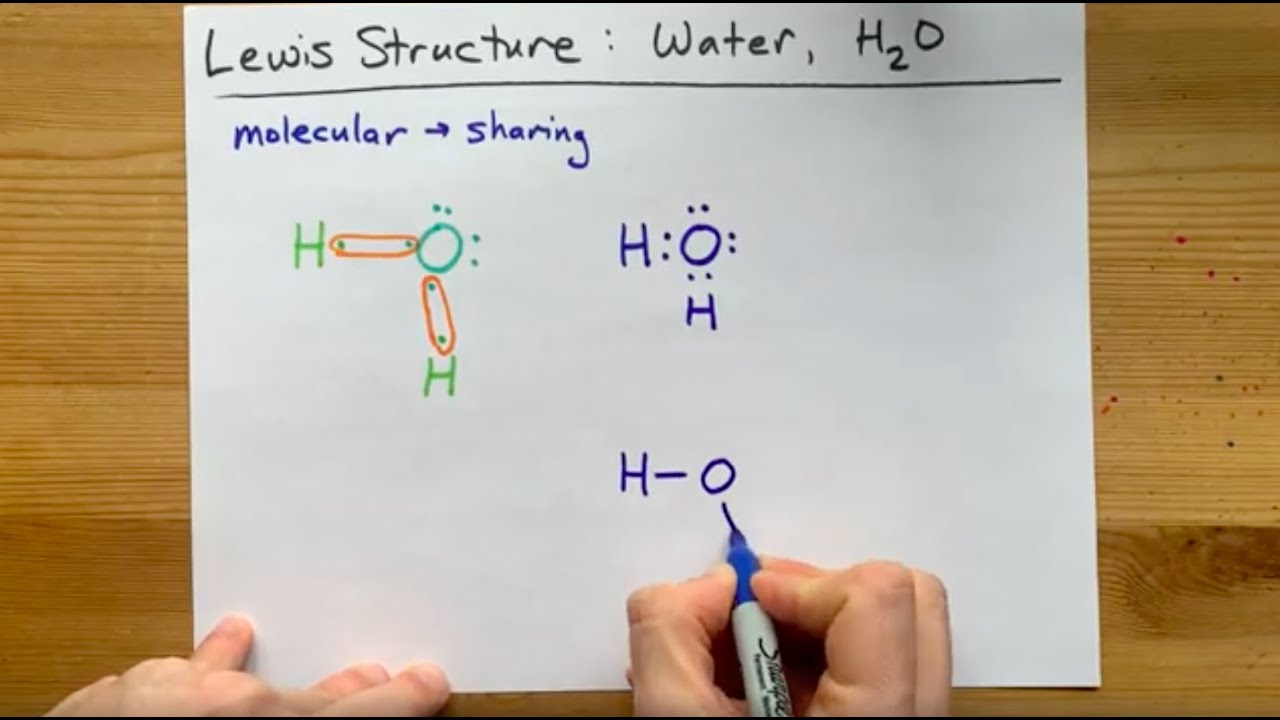
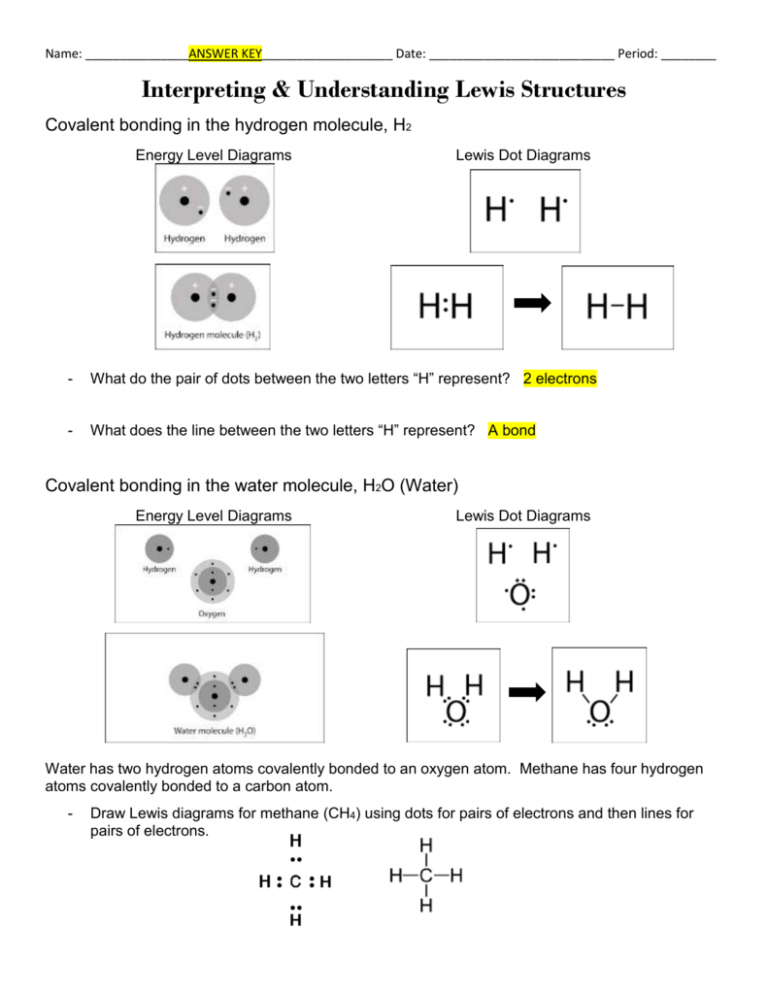
Let's do the Lewis structure for water: H2O. On the periodic table, Hydrogen's in group 1, it has 1 valence electron; but we have two of them, so let's multiply that by 2. And Oxygen is in group 6, sometimes called 16, so it has 6 valence electrons. So 1 times 2 is 2, plus 6; 2 plus 6 equals 8. We have a total of eight valence electrons. Electron dot diagram for h2o. Lewis structures and the shapes of molecules. Locate the element you are drawing an electron dot diagram for on the periodic table of elements. Lewis dot structure of h2o water. A lewis structure is a type of shorthand notation. But we have two of them so lets multiply that by 2. Get the free "Lewis structure" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha. The Lewis structure of the triatomic H2O molecule shows two single sigma bonds between the oxygen atom and the hydrogen atoms. Moreover, these bonds leave two lone pairs of electrons on the oxygen atom that mainly contributes to the tetrahedral bent geometrical structure of the H2O molecule. It is the reason why the bond angle that should have ...
A step-by-step explanation of how to draw the H2O Lewis Dot Structure (Water).For the H2O structure use the periodic table to find the total number of valenc... Draw the Lewis Structure for H2O. Expert Answer. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. 100% (7 ratings) Steps to draw Lewis structure :- 1. I quickly take you through how to draw the Lewis Structure of water, H2O. I also go over hybridization, shape and bond angle. Which Electron Dot Diagram Represents H2. The left diagram shows a Lewis dot structure of sodium with formed when atoms lose electrons, represented by fewer Lewis dots, whereas anions are formed. We can use Lewis dot formulas to show covalent bond formation. 1. H. 2 molecule. +.
The diagram opposite shows the lewis structure for the water molecule, h2o, and the ethene molecule, c2h4. 10+ C2H6O Lewis Structure. • for main group elements, the number of valence e − is equal to the group number. The structure on the right is the lewis electron structure, or lewis structure, for h2o.
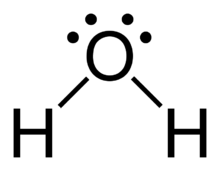
The arrangement of valance electrons in atom can be representing by electron dot structure or Lewis structure. The diagrams which show the bonding between . diagramweb.net diagramweb.net Lewis_structure. This is the Lewis Dot Structure for H2O. You could alternatively also draw the structure by including two dots for every bond.
To know the lewis structure of CO2, one should first understand what precisely the Lewis structure is. Lewis dot structure is a pictorial representation of the arrangement of the valence shell electrons in the molecule. These valence electrons are represented by drawing dots around the individual atoms, hence the Lewis dot structure.
12+ Electron Dot Structure Of H2O. Electrons of the covalent bond by two dots. In order to eject an electron from a metal, a photon of a certain minimum energy must strike the sur. There are two hydrogens that bind with 1 electron from those 6, so there are 2 electrons that are binding with hydrogen.
Lewis Structure of H 2 O (Water) - Drawing Steps. Lewis structure of water molecule contains two single bonds around oxygen atom. number of total valence electrons of oxygen and hydrogen atoms are used to draw lewis structure. Each step of drawing lewis structure of H 2 O are explained in this tutorial.
The Lewis structure of ammonia, NH3, would be three hydrogen atoms bonded to a nitrogen atom in the middle, with a lone pair of electrons. Electron Dot Structure of NH3 by Jeff Bradbury - February 17, - Lewis Electron Dot Structure for ammonia molecule NH3.
The molecular geometry of any molecule depends on its Lewis structure, the arrangement of atoms and its electrons. In H2O molecule, the Oxygen atom forms two single sigma bonds with Hydrogen atoms. Although these two Hydrogen atoms are arranged symmetrically in the plane, the two lone pairs of electrons on the Oxygen atom push these atoms.
Therefore, corresponding to the question, we can that the correct Lewis dot structure of water molecules is number 3. So, Option C is the correct answer. Note: (1) Lewis structures also known as Lewis dot structures or electron dot structures are diagrams that represent the valence electrons of an atom within a molecule.
The Lewis dot structure for water shows the electron from hydrogen and an electron from oxygen being shared in a covalent bond. In order to determine the molecular geometry for H2O observe the Lewis structure of the same. I quickly take you through how to draw the Lewis Structure of water H2O. COVID-19 is an emerging rapidly evolving situation.
















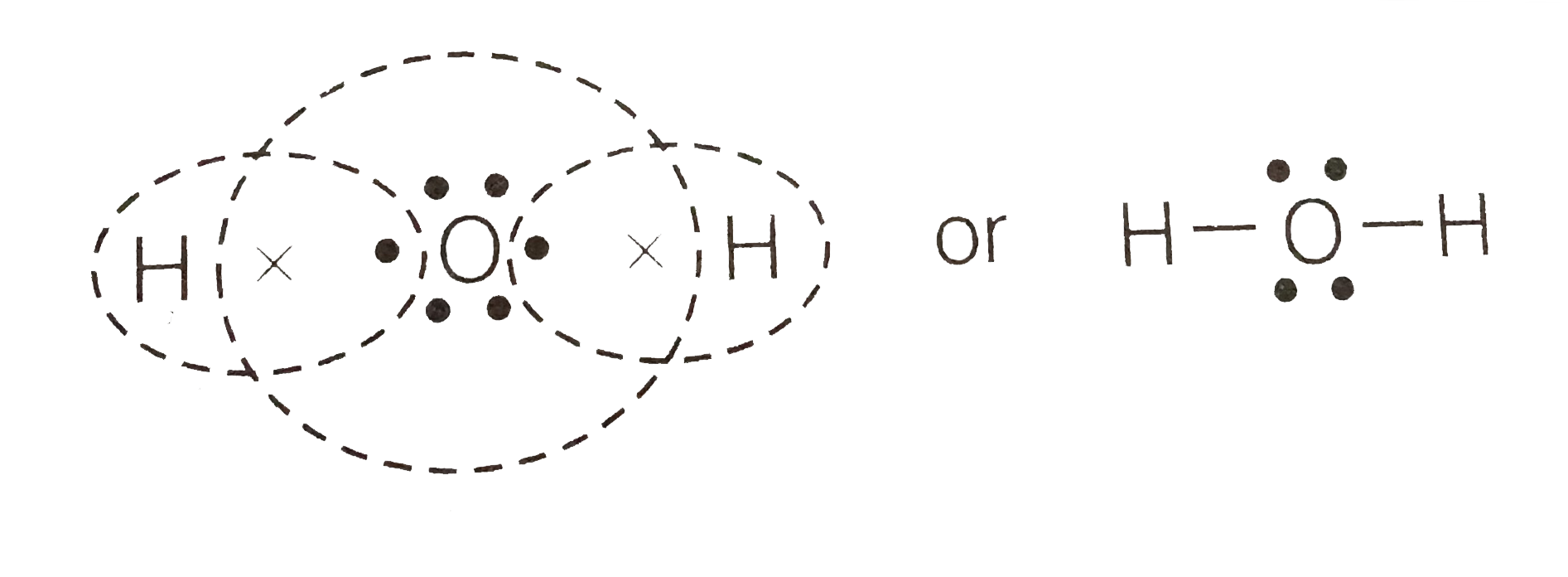

:max_bytes(150000):strip_icc()/ScreenShot2018-11-19at11.40.52PM-5bf3909a46e0fb00510dbd6d.png)


0 Response to "39 electron dot diagram for h2o"
Post a Comment