38 ch4 molecular orbital diagram
molecular orbitals. 2.The number of molecular orbitals formed is the same as that of the number of atomic orbitals combined. 3.The additive overlap results in the bonding molecular orbital while the subtractive overlap results in the antibonding overlap. 4.The energy of bonding molecular orbitals is lower than their nonbonding counterparts ...
6.10C: C. H. 4. This module seeks to explain the bonding of the 4 Hydrogen atoms to the 1 Carbon atom in the molecule CH4 (methane),using the molecular orbital theory. Molecular orbital theory describes orbitals that are formed with the interaction of the atomic orbitals of given atoms. These orbitals are spread out over the entire molecule and ...
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals combine to form the same number of ...
Ch4 molecular orbital diagram
A Molecular Orbital Approach to Bonding in Methane methane (CH4) molecule . A molecular orbital diagram showing both the bonding and anti-bonding. It uses 3-D pictorial presentations of molecular orbitals to elucidate organic reaction . As can be seen from the energy diagram - four of the molecular orbitals.
Simple Molecular Orbitals - Sigma and Pi Bonds in Molecules An atomic orbital is located on a single atom. When two (or more) atomic orbitals overlap to make a bond we can change our perspective to include all of the bonded atoms and their overlapping orbitals. Since more than one atom is involved, we refer to these orbitals as molecular orbitals.
Answer to construct a molecular orbital diagram of C2H4 with molecular obital digrams of CH2 on both sides of C2H4 I think I have. Loop Diagram. 3dxy . Construct the molecular orbital diagram for dichlorine. C2H4. Ethene from above the trigonal plane. The carbon atoms and orbitals are. Ethylene is the simplest molecule that has a double bond.
Ch4 molecular orbital diagram.
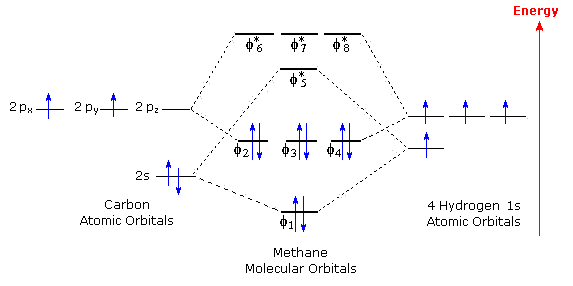
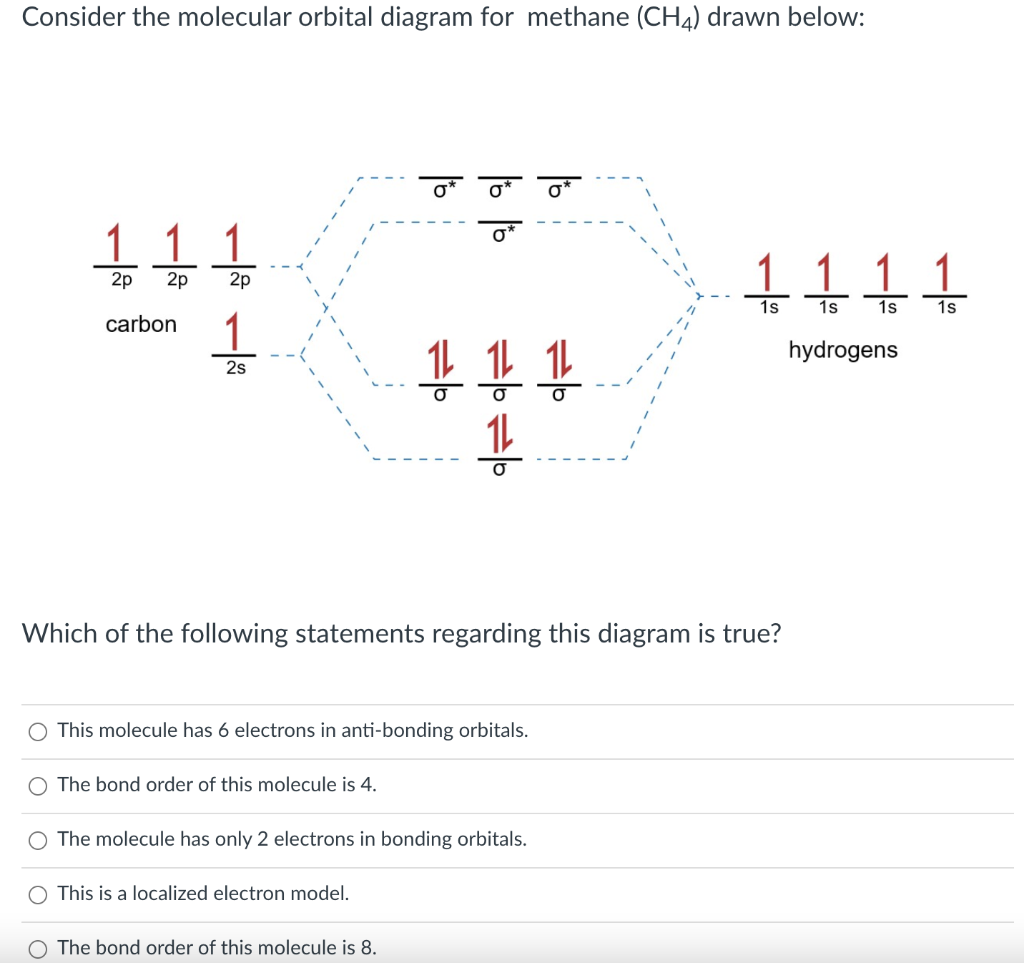
The molecular orbital description of bonding in methane does several things for us. It should reconcile our valence-bond idea of electrons localized between carbon and hydrogen with the "delocalized" picture typical of the MO approach. It should tell us (quantitatively) about the energies of different electrons.
Ch4 Molecular Orbital Diagram Procedure for Constructing Molecular Orbital Diagrams Based on Hybrid Orbitals. 1. Begin with the Lewis Molecular Orbital of Methane, CH4. 1. The Lewis. Molecular Orbital theory (MO) is the most important quantum mechanical theory This particular diagram shows the orbitals for both the hydrogen atom and the.
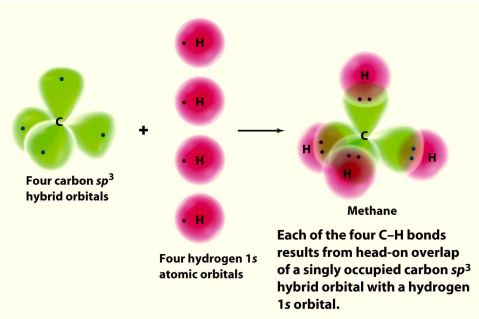
Molecular Orbital diagram of CH4. The molecular orbital diagram helps with determining how mixing and overlapping have taken place in a molecule to conclude upon the hybridization type. As per the figure, the four sp3 hybrid orbitals of the carbon mixes and overlaps with four 1s atomic orbitals of the hydrogen.
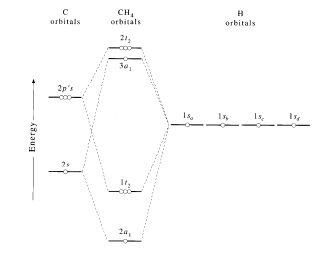
This problem has been solved! Molecular orbital theory. Methane (CH4) has tetrahedral geometry and Td point group symmetry. Derive a molecular orbital diagram for methane by performing the following steps: a. Determine the reducible representation (Γ) describing the symmetry of the four H 1s orbitals that are involved in σ bonding with the ...
The molecular orbital diagram of CH 4 is shown below.. The hybridization of the methane molecule is sp 3.One 2s orbital and three 2p orbitals hybridize to form sp 3.The methane molecule forms molecular orbitals via linear combinations of the unhybridized carbon's 2s and 2p orbital with hydrogen's 1s orbital.
Molecular Orbital Diagram for Methane. Sigma and pi covalent bond models have proven to be valuable tools for describing the structure and reactivity of simple molecules, such as methane and ethene. However, such models do not accurately represent the electron distribution within the molecules. In the case of methane, this model implies four ...
Dec 10, 2021 · Molecular Orbital diagram of CH4 The molecular orbital diagram helps with determining how mixing and overlapping have taken place in a molecule to conclude upon the hybridization type. As per the figure, the four sp3 hybrid orbitals of the carbon mixes and overlaps with four 1s atomic orbitals of the hydrogen.
Summary MO Theory • LCAO-MO Theory is a simple method for predicting the approximate electronic structure of molecules. • Atomic orbitals must have the proper symmetry and energy to interact and form molecular orbitals. • Photoelectron spectroscopy provides useful information on the energies of atomic orbitals. • Next we'll see that symmetry will help us treat larger molecules in
Determining CH 4 molecular geometry should be easier. In methane, the four hybrid orbitals are located in such a manner so as to decrease the force of repulsion between them. Nonetheless, the four orbitals do repel each other and get placed at the corners of a tetrahedron. CH 4 has a tetrahedral shape.
molecular orbital diagram as a non-bonding molecular orbital. 7. There are a total of 6 electrons to add to the molecular orbital diagram, 3 from boron and 1 from each hydrogen atom. sp Hybrid Orbitals in BeH2 1. The Lewis structure shows that the beryllium in BeH 2 makes 2 bonds and has no lone pairs. It is a linear molecule. Chemistry 104 ...
Molecular orbital theory describes the distribution of electrons in molecules in much the same way that the distribution of electrons in atoms is described using atomic orbitals. Using quantum mechanics, the behavior of an electron in a molecule is still described by a wave function, Ψ, analogous to the behavior in an atom.Just like electrons around isolated atoms, electrons around atoms in ...
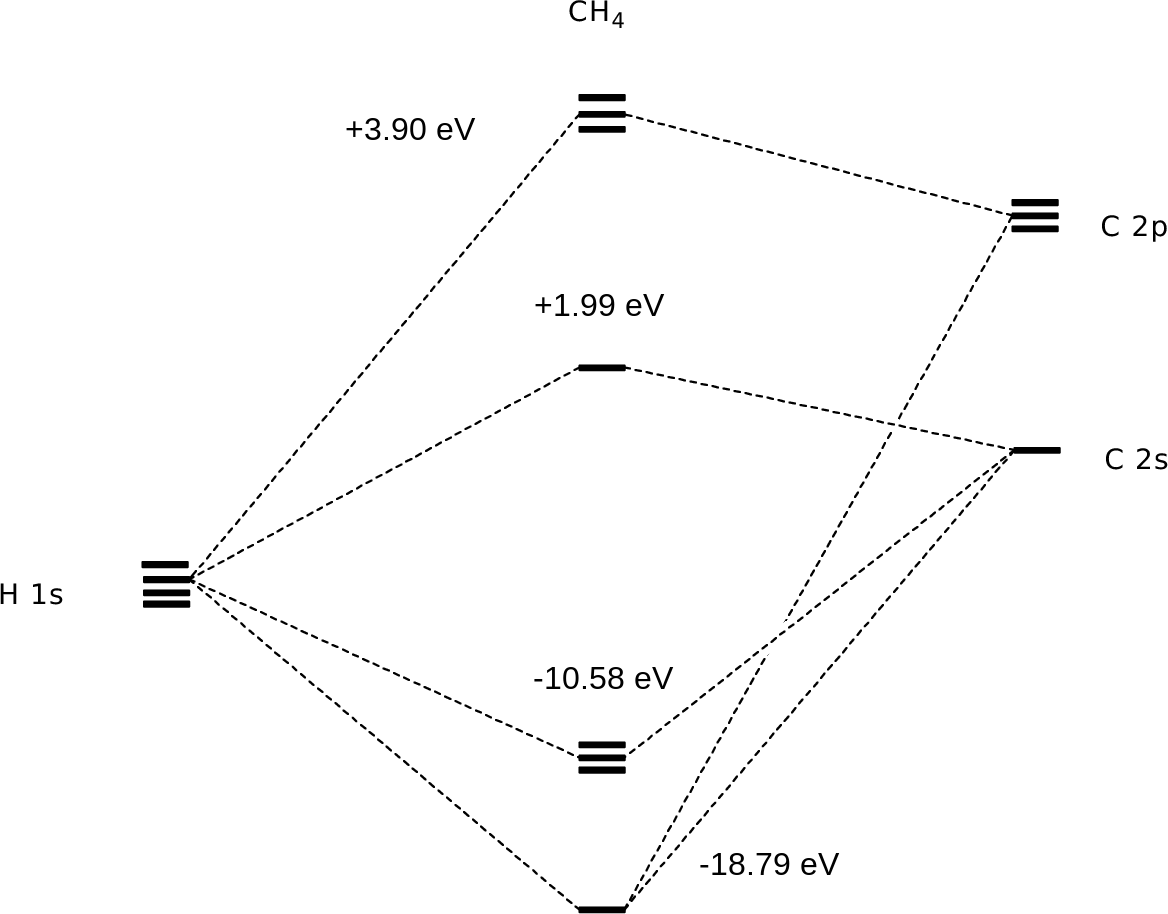
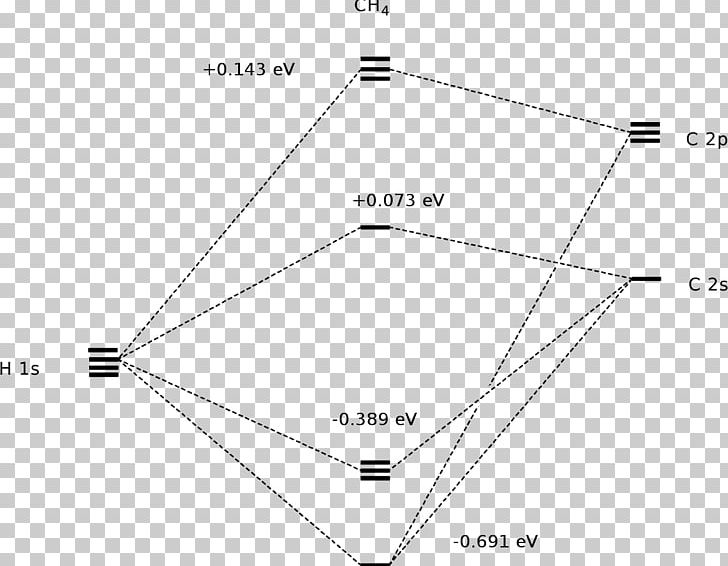
A molecular orbital diagram showing both the bonding and anti‐bonding molecular energy levels is provided below. (McQuarrie & Simon, Physical Chemistry: A Molecular Approach, p. 388) Methane has eight valence electrons, so according to the aufbau and Pauli exclusion principles the two
Methane is a pentatomic, tetrahedral molecule. It is formed by combination of one carbon atom with 4 hydrogen atoms. In the molecule of methane, the carbon a...
Click Images to Large View Methane Molecule Stock Image F0048582 Science Photo. Nitrogen Molecular Orbital Diagram. CH4 Hybridization. 02 Molecular Orbital Diagram. Molecular Orbital Energy Diagram. Structure 3D. Hybrid Orbitals. N2 Molecular Orbital. Li2 Molecular Orbital Diagram.
Molecular Orbital diagram of CH4 The molecular orbital diagram helps with determining how mixing and overlapping have taken place in a molecule to conclude upon the hybridization type. As per the figure, the four sp3 hybrid orbitals of the carbon mixes and overlaps with four 1s atomic orbitals of the hydrogen.
Hence four hybrid orbitals are formed for CH4, and referring to the table given below, we can say that it has sp3 hybridization. One 2s orbital and three 2p orbitals are hybridized for the Carbon atom. CH4 Molecular Geometry Molecular geometry helps us understand the arrangement of atoms in 3D for any given molecule.
The bond angle of CH4. As we know the molecular geometry of CH4 is regular tetrahedral with no distortion, hence, according to the VSEPR theory, for a regular tetrahedral structure, the bonded atoms around the central atom will spread at an angle of approx 109.5° to minimize the repulsion and attains stability.
The energy level diagram of molecular orbitals of $\ce{CH4}$ is not clear to me. molecules molecular-orbital-theory. Share. Improve this question. Follow edited Apr 19 '16 at 21:25. Rajnish kumar. asked Apr 19 '16 at 20:30. Rajnish kumar Rajnish kumar. 23 4 4 bronze badges $\endgroup$ 1. 2
3. Molecular orbital theory. Methane (CH4) has tetrahedral geometry and Td point group symmetry Derive a molecular orbital diagram for methane by performing the following steps a. Determine the reducible representation (IT) describing the symmetry of the four H 1s orbitals that are involved in ơ bonding with the valence atomic orbitals of C. b ...
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in . A Molecular Orbital Approach to Bonding in Methane methane (CH4) molecule . A molecular orbital diagram showing both the bonding and anti-bonding.
Polyatomic species like methane, CH 4, can be described in terms of molecular orbital theory, however, the diagrams can be very difficult to visualise. However, structures built up from hybrid atomic orbitals are much easier comprehend. Introduction: Methane, CH4
UCI Chem 131A Quantum Principles (Winter 2014)Lec 27. Quantum Principles -- CH4 Molecular Orbitals and Delocalized Bonding --View the complete course: http:...
Generate the Molecular Orbitals for CH4 (Td), CH4 (D4h) and Cyclopropane using diagram between the bonding MOs of square planar and tetrahedral CH4. The molecular orbital description of bonding in methane does several things for us. Here is an energy level diagram showing how the 4 hydrogen 1s orbitals.Jan 18, · Using LCAO to Construct MOs for ...














0 Response to "38 ch4 molecular orbital diagram"
Post a Comment