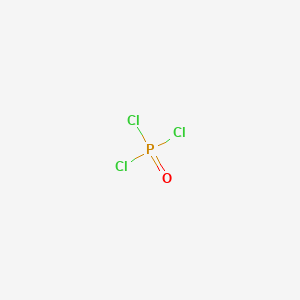
37 lewis dot diagram for pocl3
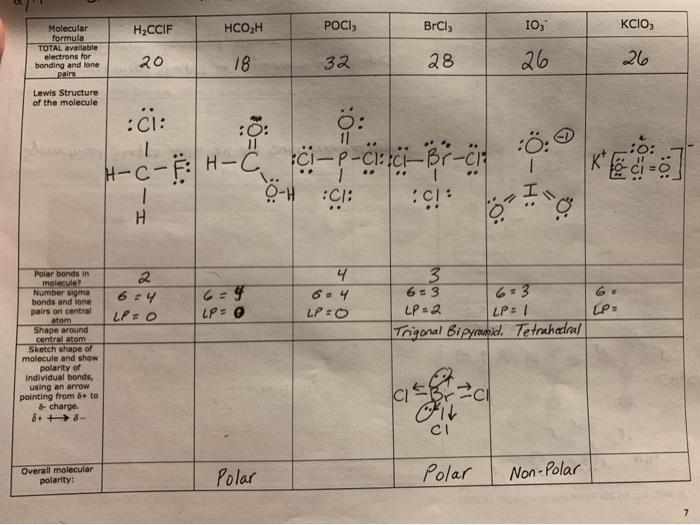
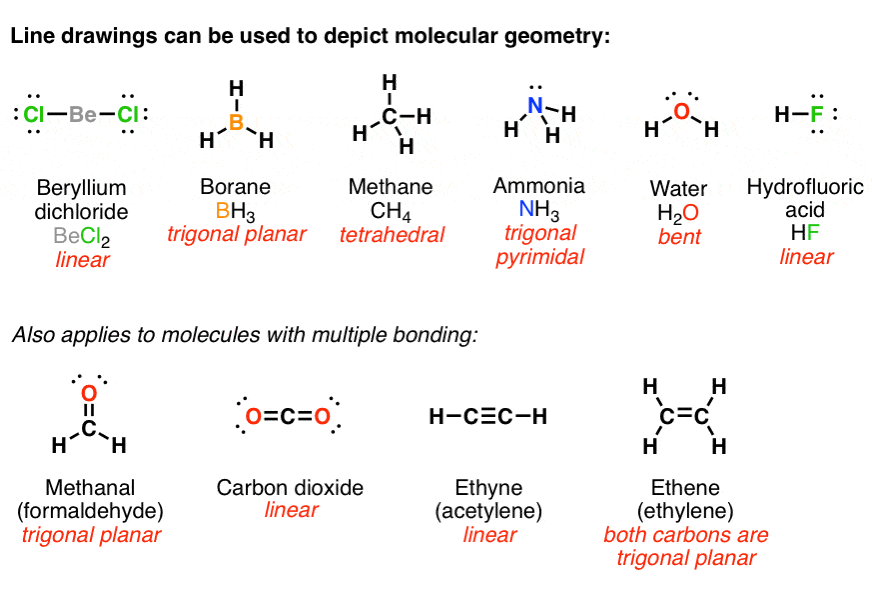
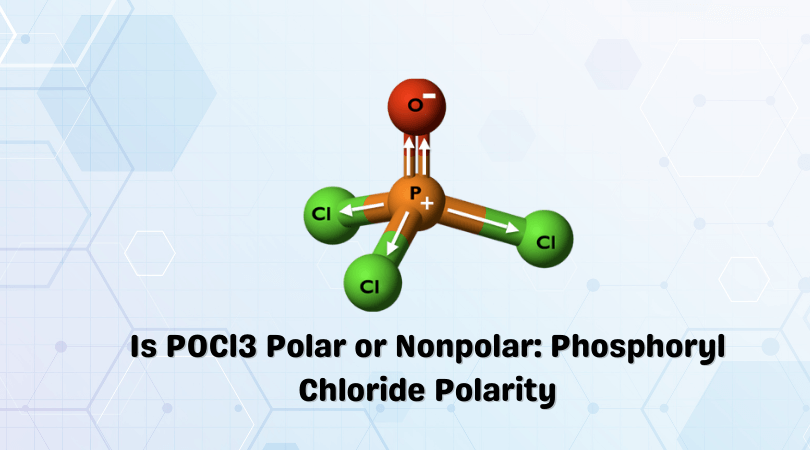
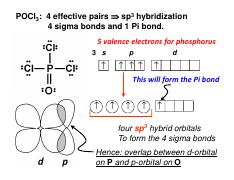
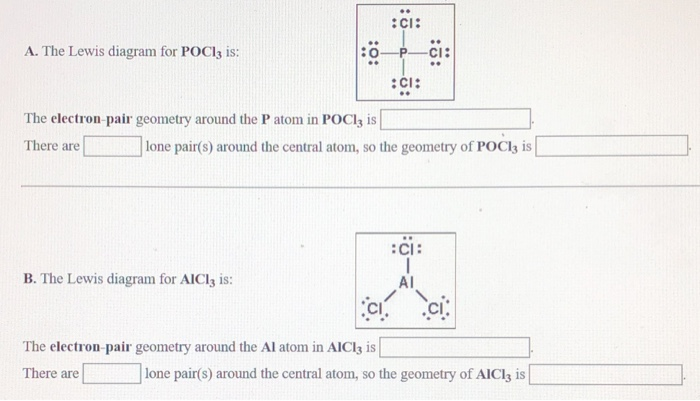
The molecular geometry of POCl3 is tetrahedral. What hybridization does this indicate for the central P atom? Why is the answer spy? asked by Chelsea on December 11, 2013 chem. Lewis Structure is one of the key terminologies to understand the chemical bonding of a molecule since it represents the molecular structure. It depends on the octet rule concept and is an extension of the electron dot diagram. Thus, to have a comprehensive idea about CS2 Lewis Structure, let us go through each step clearly and systematically.
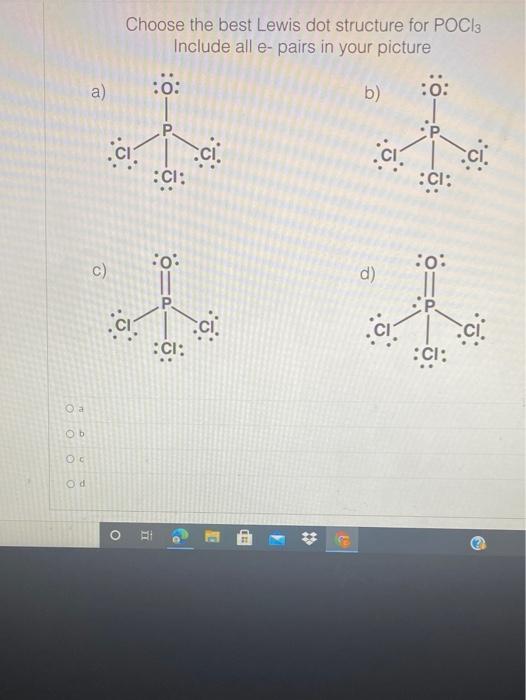
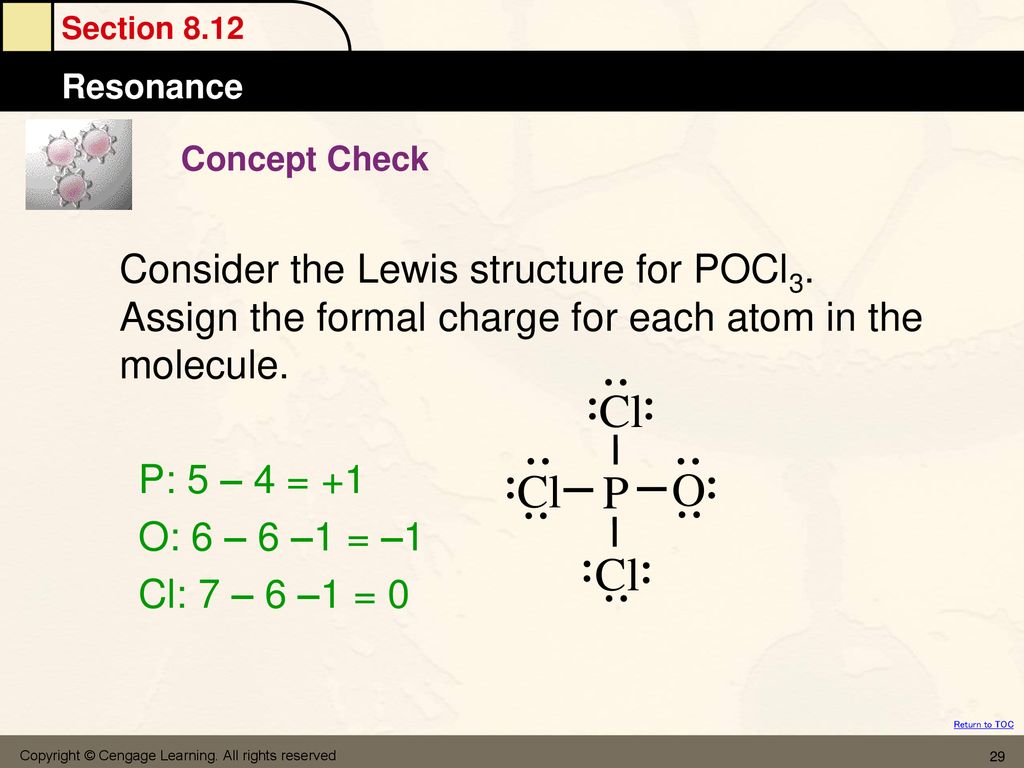
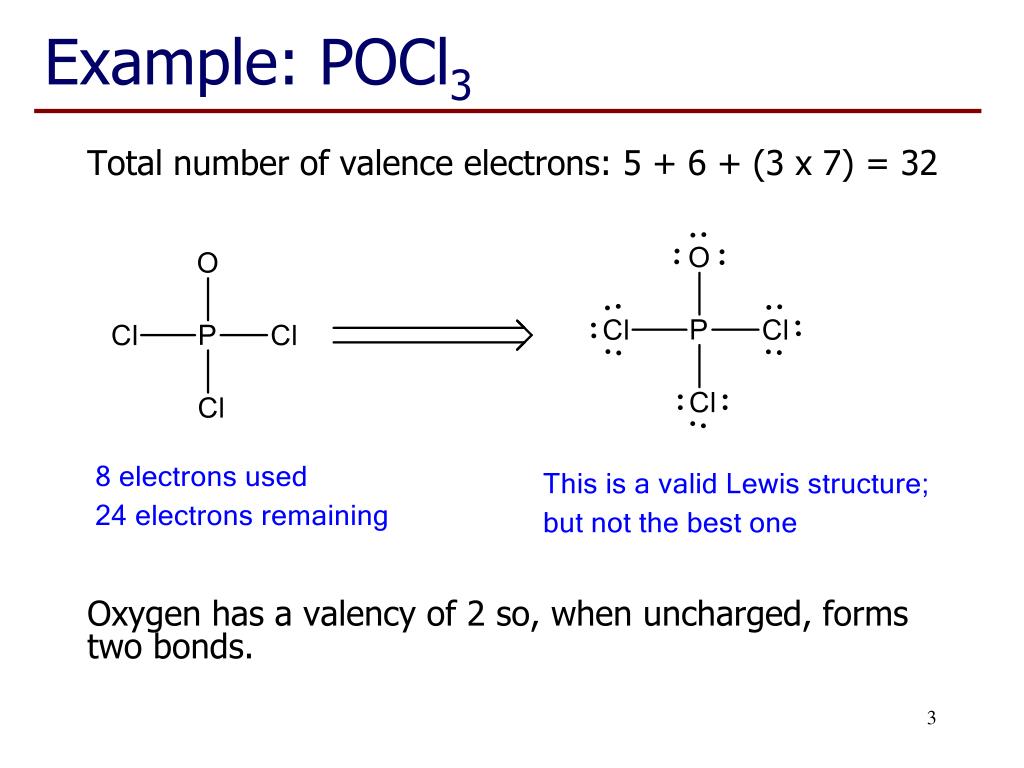
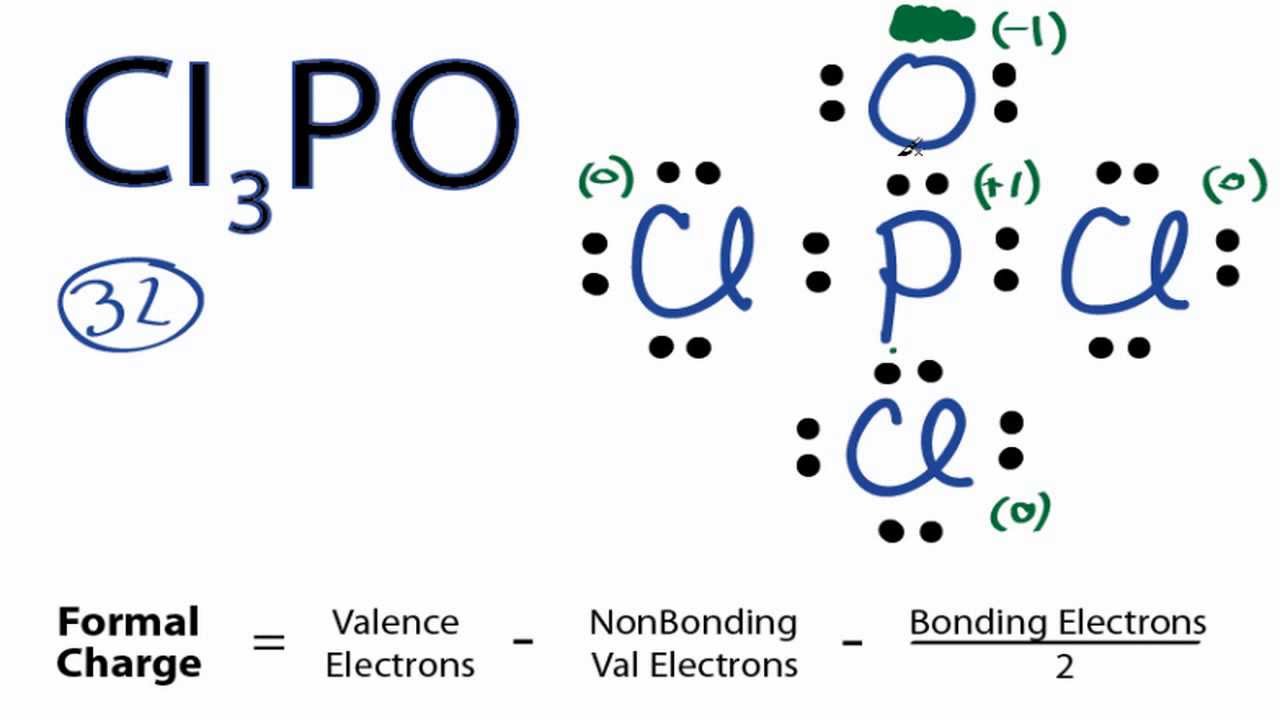
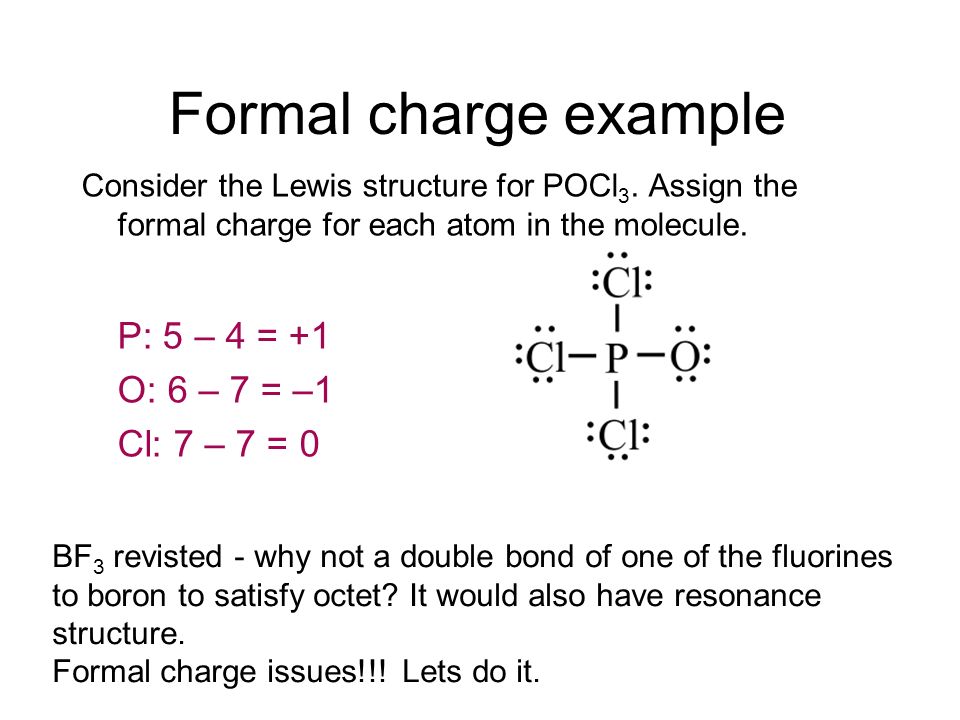
POCl3 Lewis Structure: How to Draw the Dot Structure for POCl3 Let's do the POCl3 Lewis structure. On the periodic table, Phosphorus in group 5 or 15, 5 valence electrons; 6 for the Oxygen; and then 7 for Chlorine, we do have three Chlorines.
Lewis dot diagram for pocl3
How To Draw Lewis Dot Structure For Pocl3 3/8 [Book] the evolution of retail - how retailers have adapted since the millennium A blend of police procedural and psychological thriller, "The Blacklist" has dazzled audiences for nearly a decade. Here are shows that strike a similar tone. Lewis structure of SO3. The sulfur trioxide is a tetra atomic chemical molecule where both the sulfur and three oxygen molecules bond with an equal number of valence electrons. The diagram is drawn showing dots of valence electrons around the symbol of both sulfur and oxygen atoms with lines predicting bond formation. Please feel free to start a scientific chemistry discussion here! Discuss chemistry homework problems with experts! Ask for help with chemical questions and help others with your chemistry knowledge! Moderators: expert, ChenBeier, Xen. 4945 Topics. 11503 Posts. Last post Re: esterification reactions. by raghavan. Sat Nov 27, 2021 10:20 pm.
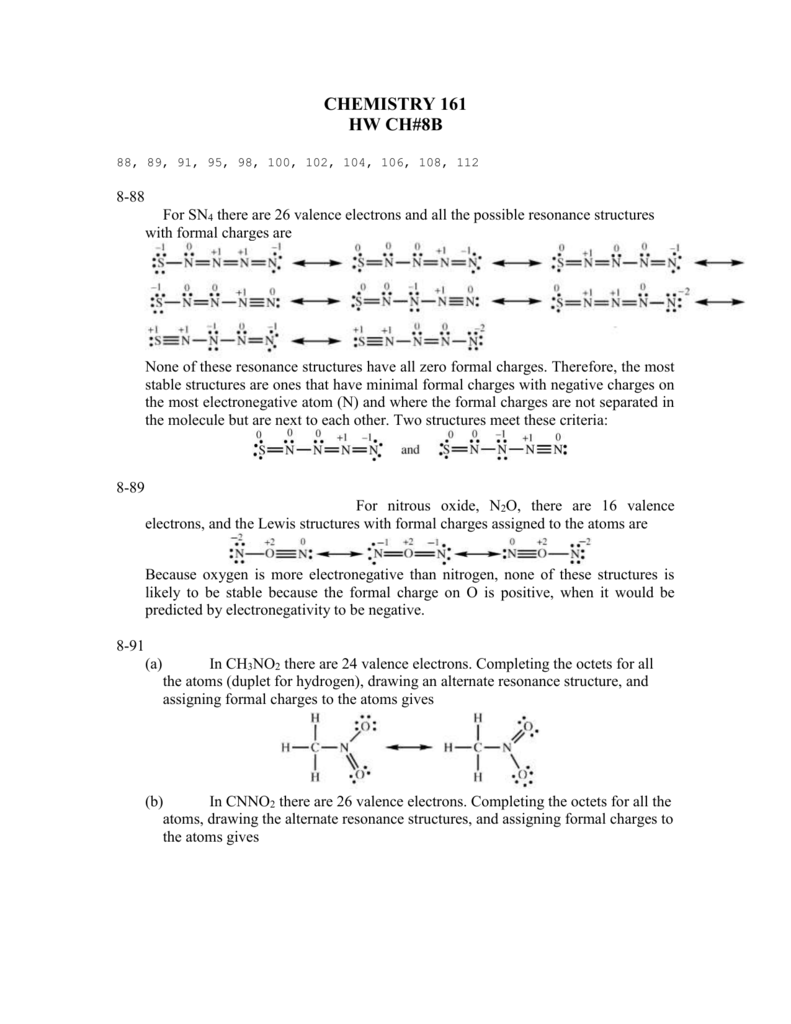
Lewis dot diagram for pocl3. BF3 Lewis Structure, Molecular Geometry, and Hybridization. Boron Trifluoride (BF3) is an inorganic compound as it lacks a carbon atom or C-H bond in the molecule. Manufactured from the reaction of boron oxides and hydrogen fluoride, the chemical compound BF3 has a pungent smell and is colorless in nature. The compound behaves differently in ... Lewis Structure of PCl5. Lewis structure of a compound is the arrangement of its underlying atom's valence shell electrons. Lewis structures make the use of dots to represent electrons and bonds between different electrons are represented through a straight line, marked at the end of which is a set of electrons. Nh2 lewis structure. Therefore it cannot be represented by a single lewis structure. NH2- and BeH2 c. NH2- Lewis Structure NH2- has a total of 8 valence electrons which are surrounded on the H-N-H structure. Total valence electrons of nitrogen and oxygen atoms and negative charge also should be considered in the drawing of NO 2-lewis structure. Unlike O 3, though, the actual structure of CO 32− is an average of three resonance structures. 1. Because carbon is the least electronegative element, we place it in the central position: 2. Carbon has 4 valence electrons, each oxygen has 6 valence electrons, and there are 2 more for the −2 charge.
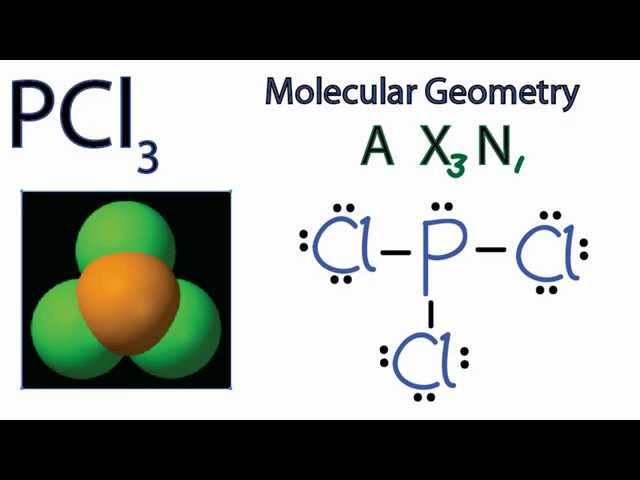
Molecular dynamics study of nanodroplet diffusion on smooth solid surfaces. NASA Astrophysics Data System (ADS) Niu, Zhao-Xia; Huang, Tao; Chen, Yong. 2018-10-01. We perform molec 9.2: The VSEPR Model. To use the VSEPR model to predict molecular geometries. To predict whether a molecule has a dipole moment. The Lewis electron-pair approach can be used to predict the number and types of bonds between the atoms in a substance, and it indicates which atoms have lone pairs of electrons. We can clearly see from the lewis diagram that in PCl3, phosphorus is forming three sigma bonds with 3 chlorine atoms. With that 2 lone pairs are present on the phosphorus atom. This concept very well explains the hybridization of PCl3 which is sp3. Another simple formula can also give us the hybridization of PCl3. Figure 3 presents the typical structure of a bifacial PERC+ cell. The front side n+ emitter region is produced with POCl3 diffusion in a tube furnace and typically passivated with a SiN x dielectric layer, which also acts as anti-reflective coating.
The Lewis structure of H2O indicates that there are four regions of high electron density around the oxygen atom: two lone pairs and two chemical bonds: ... POCl3 (P is the central atom) (c) Cl2SeO (Se is the central atom) ... Draw the Lewis electron dot structures for these molecules, including resonance structures where appropriate: (a ... Please feel free to start a scientific chemistry discussion here! Discuss chemistry homework problems with experts! Ask for help with chemical questions and help others with your chemistry knowledge! Moderators: expert, ChenBeier, Xen. 4945 Topics. 11503 Posts. Last post Re: esterification reactions. by raghavan. Sat Nov 27, 2021 10:20 pm. Lewis structure of SO3. The sulfur trioxide is a tetra atomic chemical molecule where both the sulfur and three oxygen molecules bond with an equal number of valence electrons. The diagram is drawn showing dots of valence electrons around the symbol of both sulfur and oxygen atoms with lines predicting bond formation. How To Draw Lewis Dot Structure For Pocl3 3/8 [Book] the evolution of retail - how retailers have adapted since the millennium A blend of police procedural and psychological thriller, "The Blacklist" has dazzled audiences for nearly a decade. Here are shows that strike a similar tone.
















0 Response to "37 lewis dot diagram for pocl3"
Post a Comment