35 dot-and-cross diagram
XeF4 Lewis Structure. Now that we know the valence electrons of Xenon Tetrafluoride, it will be easier for you to draw its Lewis structure. This Lewis dot structure is a pictorial representation of valence electrons around individual atoms in a molecule along with the bond it forms. Phosphorus at Chemical schematron.org Basic Information °C Number of Protons/Electrons: 15 , Number of.Bohr diagram for phosphorus furthermore atoms as well as electron configurations in addition plutonium electron diagram further chlorine electron dot diagram also carbon dioxide dot and cross diagram along with silicon atom model ...
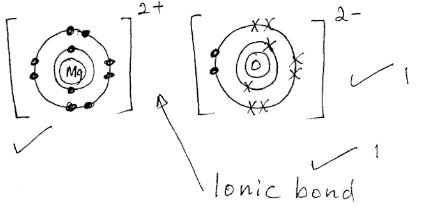
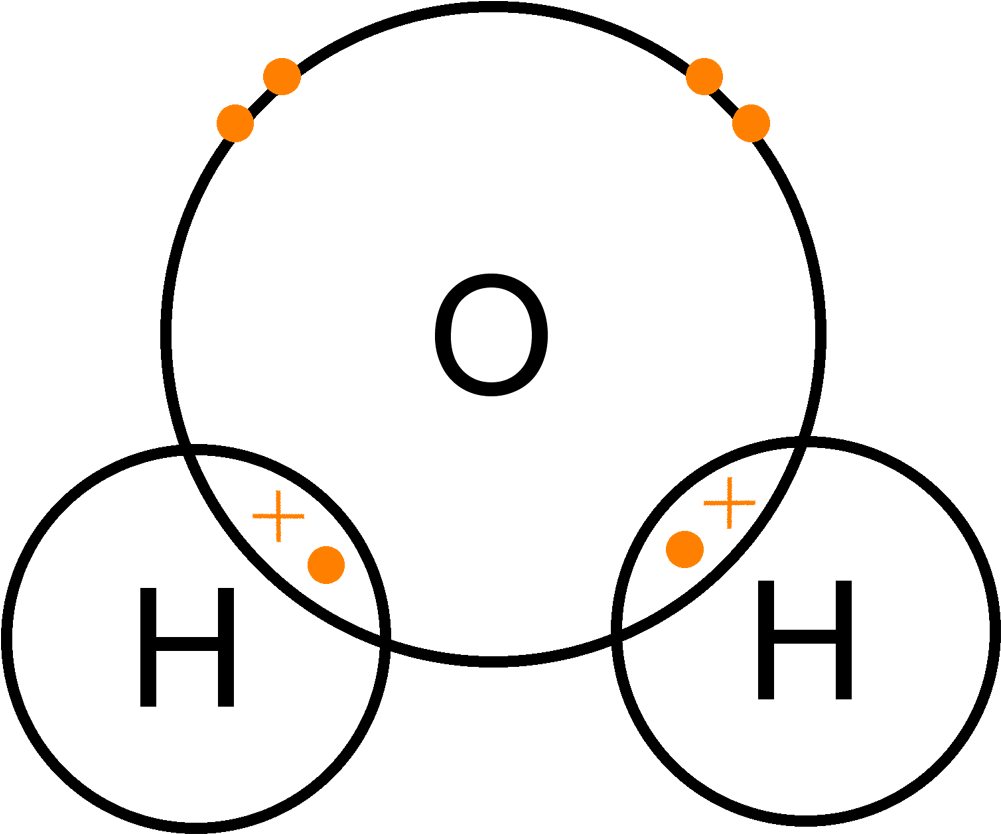
View more ». Draw dot-and-cross diagram to represent the electron transfer that takes place. I 4. Draw Lewis Structure (dot and cross) diagrams to represent: (a) chlorine molecule, Cl; (b) water molecule, H:0 (c) ammonia molecule, NH, Page 3 of 4 622 words & English (United States 3. Element X has one electron in its outer shell and Element Y ...

Dot-and-cross diagram
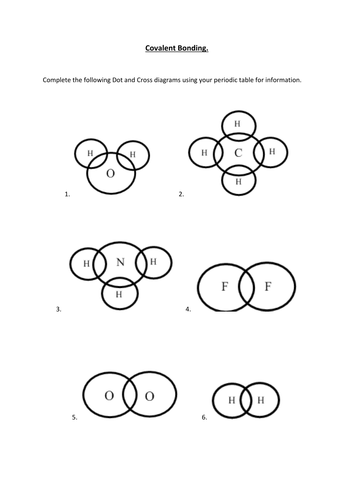
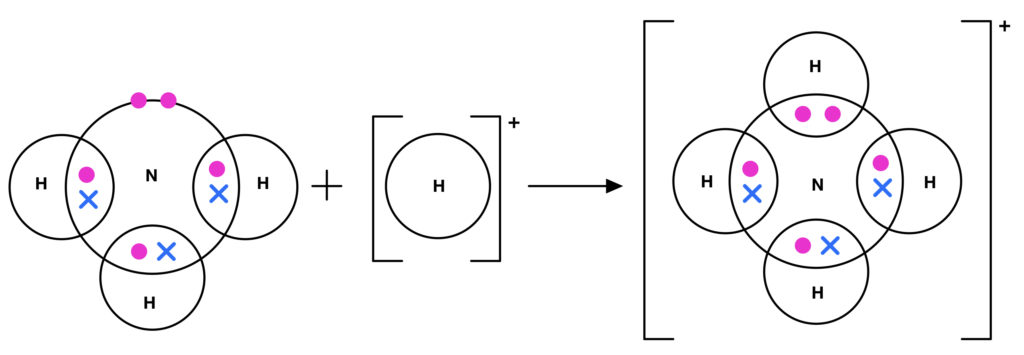
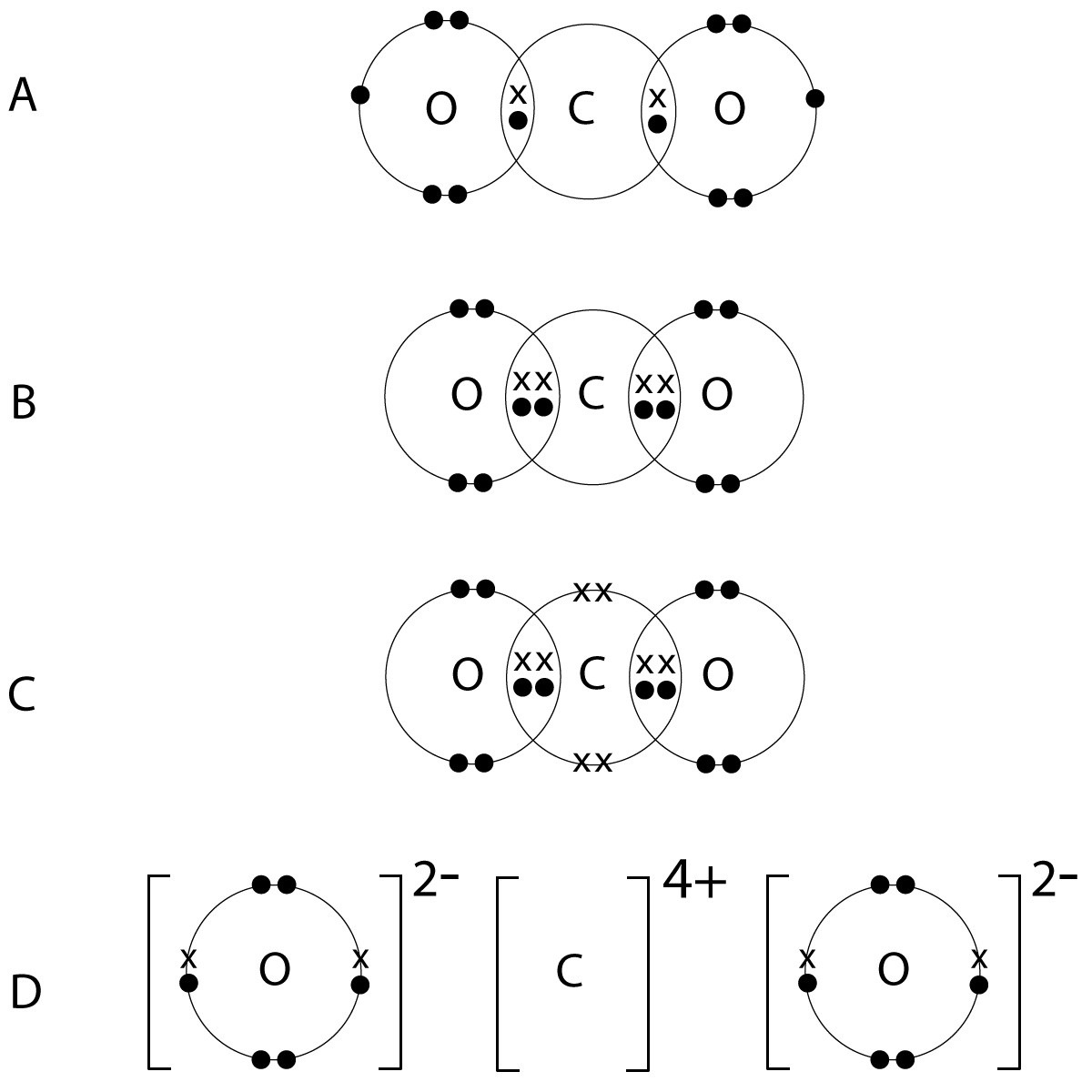
Resource includes: step by step guide to drawing dot and cross diagrams. worked examples for single covalent bonds (fluorine, F2) and multiple covalent bonds (carbon dioxide, CO2) structured activities for students to complete for drawing dot and cross diagrams for: hydrogen, hydrogen bromide, chlorine, methane, ammonia, water, oxygen, nitrogen ... A Lewis Structure is a depiction of the arrangement of electrons in the standalone atoms of an element. In the Lewis Structure, electrons are depicted as dots. A bond between two electrons is represented by a line marked by a dot at both ends, involving the participating electrons. Arrow diagram, we would Draw this with an arrow from the lone! Class X dot and cross diagram for ammonium ion by navnit40 ( -4,939 points this can be seen in our dot-and-cross diagram H⁺ an. Structure to the nitrate ion but carbon has only four outer shell electrons. cross diagrams the!
Dot-and-cross diagram. SF6 Molecular Geometry, Lewis Structure, Shape, and Polarity. July 23, 2021. Posted by Priyanka. 16 Apr. Sulfur hexafluoride or SF6 is an inorganic, greenhouse gas. It is non-flammable, odourless, and colourless, and is an excellent insulator. It is a hypervalent octahedral molecule that has been an interesting topic of conversation among ... The goal when identifying model boundaries and size for each microservice isn't to get to the most granular separation possible, although you should tend toward small microservices if possible. Instead, your goal should be to get to the most meaningful separation guided by your domain knowledge. The emphasis isn't on the size, but instead on ... Covalent Bond: Definition, Types, Properties, Solved Examples. Covalent Bond is a bond formed by the sharing of electrons present in the valence shell of the atoms. It holds the atoms within an individual molecule together. We are told not to touch electrical appliances with bare wet hands. Learn more. Some of the worksheets below are Ionic Bonding Worksheets, learn the the four major types of bonds viz, ionic bonds, metallic bonds, covalent bonds, intermolecular forces with several exam style questions and answers. Once you find your worksheet (s), you can either click on the pop-out icon or download button to print or download ...
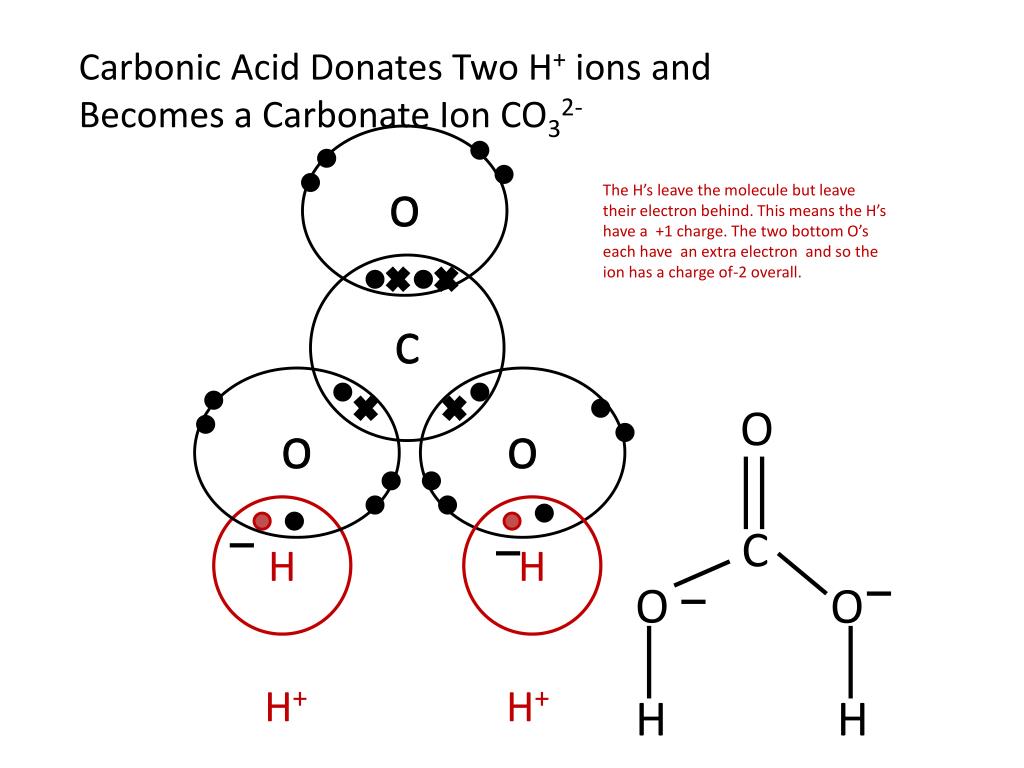
This worksheet clearly explains how to draw dot and cross diagrams for covalent compounds using cl2 as an... Read More . Worksheet. Preschool Math Worksheets Counting Work Bun. November 25, 2021. Number recognition is a building block to success in math. More advanced worksheets are provided in our grades 1 6 free math worksheet section. This type of bonding would be a covalent bond. Two combinations of atoms can produce this type of bonding: nonmetal/nonmetal or metalloid/nonmetal. In this class, we will not discuss the option of metallic bonding which is a form of covalent bonding. Figure 4.8. 1: Sharing is caring, especially for atoms that participate in covalent bonding. Answer: 2 📌📌📌 question Complete the dot and cross diagram, in Figure 2, for tetrachloromethane, CCl4 - the answers to estudyassistant.com Dot-and-cross diagrams including molecular ions • The molecular ions have covalent bonds in but have gained or lost electrons from the elements within them. • That means they bind ionically to other oppositely charged ions: • Calcium hydroxide (Qu10). 2 Clue for Q9: Cyanide is a molecular ion: - CΞN H O - Ca 2+
By default, DOT assumes the UTF-8 character encoding. It also accepts the Latin1 (ISO-8859-1) character set, assuming the input graph uses the charset attribute to specify this. For graphs using other character sets, there are usually programs, such as iconv, which will translate from one character set to another. The diagram below shows the arrangement of the atoms in each layer, and the way the layers are spaced. Notice that you cannot really draw the side view of the layers to the same scale as the atoms in the layer without one or other part of the diagram being either very spread out or very squashed. In that case, it is important to give some idea ... Drawing dot-cross diagram is a fundamental and essential technique for us to determine the lewis structure and shape of molecules and polyvalent ions. For A ...Oct 30, 2018 · Uploaded by Chemistry Guru - #1 JC, A Level, H2 Chemistry Tuition Complete the dot and cross diagram, in Figure 2, for tetrachloromethane, CCl4 - 20392994
Dot product is also known as scalar product and cross product also known as vector product. Dot Product - Let we have given two vector A = a1 * i + a2 * j + a3 * k and B = b1 * i + b2 * j + b3 * k. Where i, j and k are the unit vector along the x, y and z directions. Then dot product is calculated as dot product = a1 * b1 + a2 * b2 + a3 * b3.
Draw dot-and-cross diagram to represent the electron transfer that takes place. I 4. Draw Lewis Structure (dot and cross) diagrams to represent: (a) chlorine molecule, Cl; (b) water molecule, H:0 (c) ammonia molecule, NH, Page 3 of 4 622 words &...
Answer (1 of 3): I modified the picture from this post: What's the MOT diagram of O2 +2 ion? and modified it to be O2 2+ (since sadly enough I am about as advanced with artistic programs on pc as a rock). How you basically do these questions is by first drawing the empty AO and MO, then counting ...
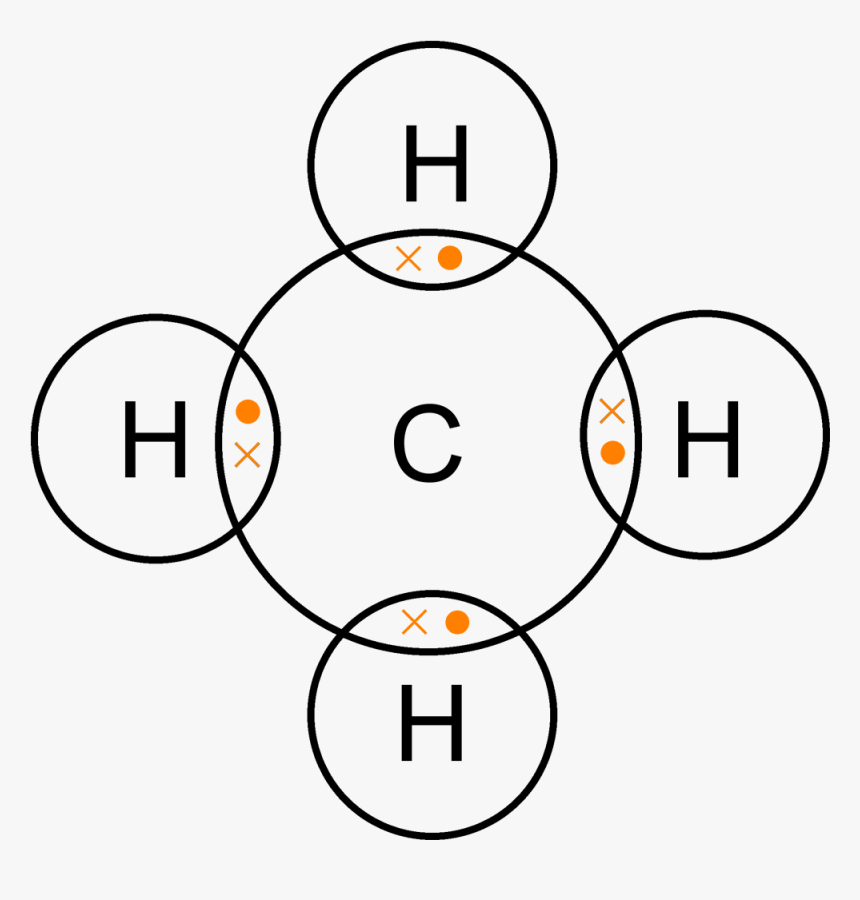
Draw a dot (.) and cross (X) diagram to show the bonding in the molecules of : (Atomic number of carbon is 6, Oxygen is 8 and hydrogen is 1) asked Aug 18 in Chemistry Form 2 by anony mous structure and bonding
The wheel rotates in the clockwise (negative) direction, causing the coefficient of the curl to be negative. Figure 16.5.6: Vector field ⇀ F(x, y) = y, 0 consists of vectors that are all parallel. Note that if ⇀ F = P, Q is a vector field in a plane, then curl ⇀ F ⋅ ˆk = (Qx − Py) ˆk ⋅ ˆk = Qx − Py.
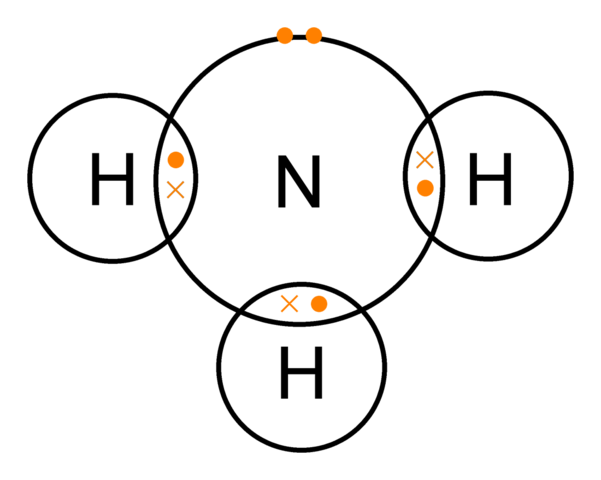
For more on drawing dot and cross diagrams for covalent bonds click here.. The electrons from the hydrogen atoms are shown as crosses and the electrons from the nitrogen atom are shown as dots. As it is an ion, square brackets are drawn around the diagram with the charge of the ion shown on the outside.
Given two linearly independent vectors a and b, the cross product, a × b, is a vector that is perpendicular to both a and b and thus normal to the plane containing them. the dot product of the … 1.5: The Dot and Cross Product - Mathematics LibreTexts
Scalar product or Dot product; Vector Product or Cross product. Scalar Product/Dot Product of Vectors. The resultant of scalar product/dot product of two vectors is always a scalar quantity. Consider two vectors a and b. The scalar product is calculated as the product of magnitudes of a, b, and cosine of the angle between these vectors.
Diagram is a way of expressing some underlying logic, convention is a way of drawing the diagram - it is the underlying logic that is important, everything else is secondary. (Actually even this "logic" is a simplification of reality and is in a way secondary to what is really going on in molecules). Sep 23, 2021. #9. lioric. 280. 5. Borek said:
10+ Ionic Bonding Diagram. If you draw the electron dot. A simple view of ionic bonding. Dot and cross diagrams - Ionic bonding - YouTube from i.ytimg.com Ionic bond strength and lattice energy. The electron dot diagrams show the nature of the electron transfer that takes. An ionic compound is…
Predicting phosphine reactivity with one simple metric. The %Vbur (min) descriptor is uniquely capable of predicting spectroscopic ligation state outcome, revealing reactivity cliffs in ...
N2H2 Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram. Dinitrogen dihydride has the chemical formula of N2H2. This compound is most commonly known as diazene or diimide. It is a yellowish-colored gas having both cis and trans isomers. It can be prepared from the decarboxylation of azodicarboxylic acid ( (NCOOH)2 ).
Arrow diagram, we would Draw this with an arrow from the lone! Class X dot and cross diagram for ammonium ion by navnit40 ( -4,939 points this can be seen in our dot-and-cross diagram H⁺ an. Structure to the nitrate ion but carbon has only four outer shell electrons. cross diagrams the!
A Lewis Structure is a depiction of the arrangement of electrons in the standalone atoms of an element. In the Lewis Structure, electrons are depicted as dots. A bond between two electrons is represented by a line marked by a dot at both ends, involving the participating electrons.
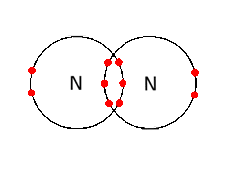
Resource includes: step by step guide to drawing dot and cross diagrams. worked examples for single covalent bonds (fluorine, F2) and multiple covalent bonds (carbon dioxide, CO2) structured activities for students to complete for drawing dot and cross diagrams for: hydrogen, hydrogen bromide, chlorine, methane, ammonia, water, oxygen, nitrogen ...




















0 Response to "35 dot-and-cross diagram"
Post a Comment