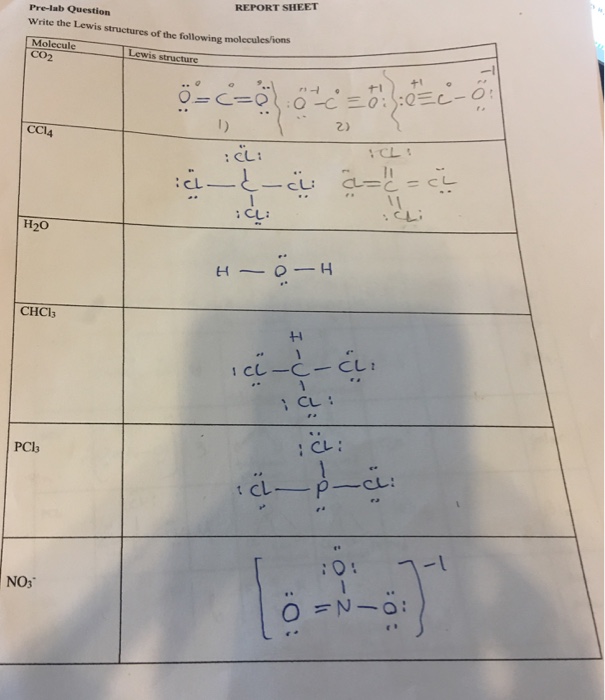
40 lewis dot diagram for ccl4
Lewis dot structure of atoms link. A ccl4 lewis structure is a diagram that represents the electron configuration of covalently bonded compounds. What are lewis dot structures used for? Lewis structure by bond determination. 16 rules for lewis structures 2. The central atom determine the geometry of the species. CCl4 Lewis Structure | Science Trends May 14, 2021 Posted by artnews No Comments. Lewis dot structures help predict molecular geometry. This example problem shows the steps to draw a structure where an atom violates the octet rule. "Lewis Structures and the Octet Rule. ... How to draw the Lewis Dot Structure for NO+.
With the help of the ClF3 Lewis dot structure, we know chlorine is the central atom that contains 2 lone pairs and is attached to 3 bonded atoms. So, the ClF3 formula becomes AX3N2 . According to the AX3N2 formula, ClF3 molecular geometry is T-shaped and electron geometry is trigonal pyramidal .

Lewis dot diagram for ccl4
Hbr lewis structure how to draw the dot structure for hbr. 11+ Ch3Br Lewis Structure. Draw lewis structures of all of the important resonance states for the following molecules or ions. Nf3 hbr sbr2 ccl4 write a lewis structure for each molecule. We start by writing symbols that contain the correct number of valence electrons for the atoms in the. Lewis dot structure answer sheet write the lewis dot structures for each of the following. Lewis dot structures and molecule geometries worksheet answer key 3 4. Then name their electron arrangement shape and bond angles. Ch3cl sif5 e ii clf3 t answer key 4 0 6 6 3 c o. Lewis dot structure worksheet author. (a) Explain what is the valence electron is and why the Lewis symbol for an atom only accounts for electrons in the valence shell of the atom. (b) Sketch the Lewis Dot Structure for each of the following compounds and state the molecular geometry. i. BeF2 ii. BCl3 iii. CCl4 iv. PBr5 (c) What is the octet rule and how it is achieved?
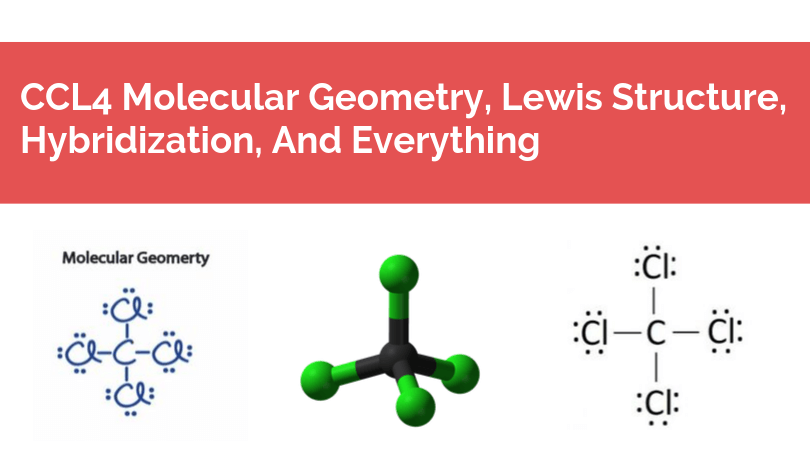
Lewis dot diagram for ccl4. As an example, let's use carbon tetrachloride, CCl4. The single carbon atom contains four valence electrons, and each of the four chlorine atoms contains seven ... The Lewis Structure, also called electron dot structure, is an essential version of chemical bonding where we utilize the valence electron concept to schematically illustration two-dimensional number of a given molecule. Since we have central atom and complete valence electron number, we will locate out exactly how electrons are located to ... Lewis Structure of PCl5. Lewis structure of a compound is the arrangement of its underlying atom's valence shell electrons. Lewis structures make the use of dots to represent electrons and bonds between different electrons are represented through a straight line, marked at the end of which is a set of electrons. Sticks or straight lines represent the bonds. While the dots represent the non-bonding electrons. Lewis theory is based on the octet rule, which states that an atom should have eight electrons in its outer shell to be stable. For the Lewis structure of CCl4 first, let's calculate the total valence electrons.
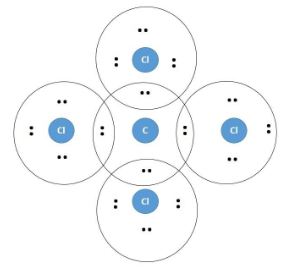
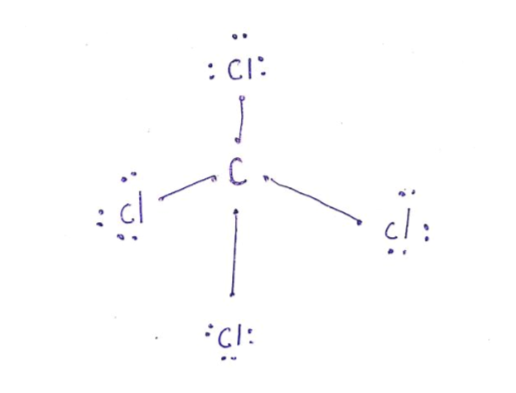
A Lewis dot structure illustrates the sharing of electrons between atoms in covalent or polar covalent bonds (both explained in this page of my website). The dots in a Lewis dot structure represent an atom's valence electrons and the placement of the dots indicate how the electrons are distributed in a molecule. What is the Lewis dot structure for CCl4? Lewis Dot of Carbon TetraChloride CCl4 TetraChloromethane. In the carbon tetrachloride molecule, four chlorine atoms are positioned symmetrically as corners in a tetrahedral configuration joined to a central carbon atom by single covalent bonds. Because of this symmetrical geometry, CCl 4 is non-polar. 7 Why does CCl4 have no dipole moment. Drawing the Lewis structure of PCl5. P does not follow the octet rule. Why does PCl5 break the Octet Rule. Draw a Lewis dot structure for the formaldehyde molecule CH2O. Those atoms can be the same element as when oxygen bonds with itself to form O2 or with different elements such as water H2O ... The total lone pair present in the CCl4 lewis dot structure is 12. Lone pairs of electrons do not involve in chemical bonds and it is represented as a dot in the lewis diagram. To draw the stable Carbon tetrachloride lewis structure, we have to represent the valence electron of each atom within a molecule.
The lewis structure for ccl4 is similar to cf4. Source: www.docbrown.info. To draw the lewis structure for so2, we have to find out the valence electrons of sulfur and oxygen first.we express valence electrons as dots in lewis dot structure. Source: s3.studylib.net. So would the right structure have p in the middle, covalently bonding to 4 ... 0000005679 00000 n 1.A Lewis electron-dot formula (Lewis structure) is identical to a structural formula. :[29], Treatment of the double salt with hydrogen sulfide gives CsCl:[29], High-purity CsCl is also produced from recrystallized It is closely related to Lewis structure and it is an integral part of organic chemistry. A Lewis structure is a pictorial diagram showing how electrons get distributed around an atom. Lewis structures are drawn to help one understand or predict how many types of bonds can be formed around a given atom. The drawing is started by determining types of covalent bonds that are formed after combining atoms. CCl4 lewis's structure is made up of one carbon atom that is situated at the middle position and four chlorine atoms that are at the surrounding position. The ...Total Valence electron of CCl4: 32The formal charge of CCl4: 0Molecular geometry of CCl4: TetrahedralName of Molecule: Carbon tetrachloride
CCl4 Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram Carbon Tetrachloride is a chemical that is produced and is not available naturally. In its natural state, it is a colorless liquid chemical with a little sweet smell like ether.
In the carbon tetrachloride molecule, four chlorine atoms are positioned symmetrically as corners in a tetrahedral configuration joined to a central carbon atom ...Sep 22, 2010 · Uploaded by kentchemistry.com
XeF4 Lewis Structure. Now that we know the valence electrons of Xenon Tetrafluoride, it will be easier for you to draw its Lewis structure. This Lewis dot structure is a pictorial representation of valence electrons around individual atoms in a molecule along with the bond it forms.
Lewis dot structure worksheet pdf · lewis dot structure worksheett answers pdf . Chemical formula, total number of valence electrons, lewis dot structure. Basics of bonding & lewis dot structures. Identify the type of bond described for each of the following as ionic, polar covalent, nonpolar covalent, or metallic.
Nov 12, 2018 — A CCL4 Lewis structure is a diagram that represents the electron configuration of covalently bonded compounds. Lewis structures are meant to ...
What is the Lewis dot structure of CCl4? Lewis Dot of Carbon TetraChloride CCl4 TetraChloromethane. In the carbon tetrachloride molecule, four chlorine atoms are positioned symmetrically as corners in a tetrahedral configuration joined to a central carbon atom by single covalent bonds. Because of this symmetrical geometry, CCl 4 is non-polar.
A CCL4 Lewis structure is a diagram that represents the electron configuration of covalently bonded compounds. Lewis structures are meant to provide a visualization of the atomic structure and the distribution of electrons in a given chemical compound. A regular atom of carbon has 4 lone electrons in its outer shell.
Whether you call them Lewis structures, electron dot structures, Lewis electron dot structures, Lewis dot structures, Lewis dot diagrams, or Lewis dot formulas, it wouldn't matter because the use is exact.
(a) Explain what is the valence electron is and why the Lewis symbol for an atom only accounts for electrons in the valence shell of the atom. (b) Sketch the Lewis Dot Structure for each of the following compounds and state the molecular geometry. i. BeF2 ii. BCl3 iii. CCl4 iv. PBr5 (c) What is the octet rule and how it is achieved?
Lewis dot structure answer sheet write the lewis dot structures for each of the following. Lewis dot structures and molecule geometries worksheet answer key 3 4. Then name their electron arrangement shape and bond angles. Ch3cl sif5 e ii clf3 t answer key 4 0 6 6 3 c o. Lewis dot structure worksheet author.
Hbr lewis structure how to draw the dot structure for hbr. 11+ Ch3Br Lewis Structure. Draw lewis structures of all of the important resonance states for the following molecules or ions. Nf3 hbr sbr2 ccl4 write a lewis structure for each molecule. We start by writing symbols that contain the correct number of valence electrons for the atoms in the.


















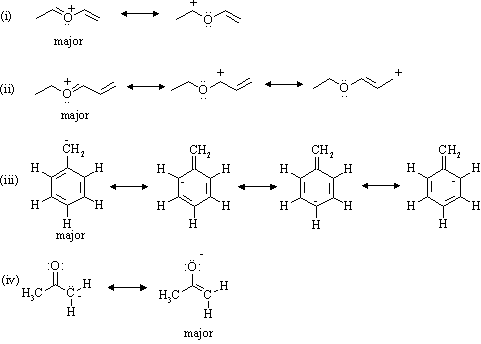

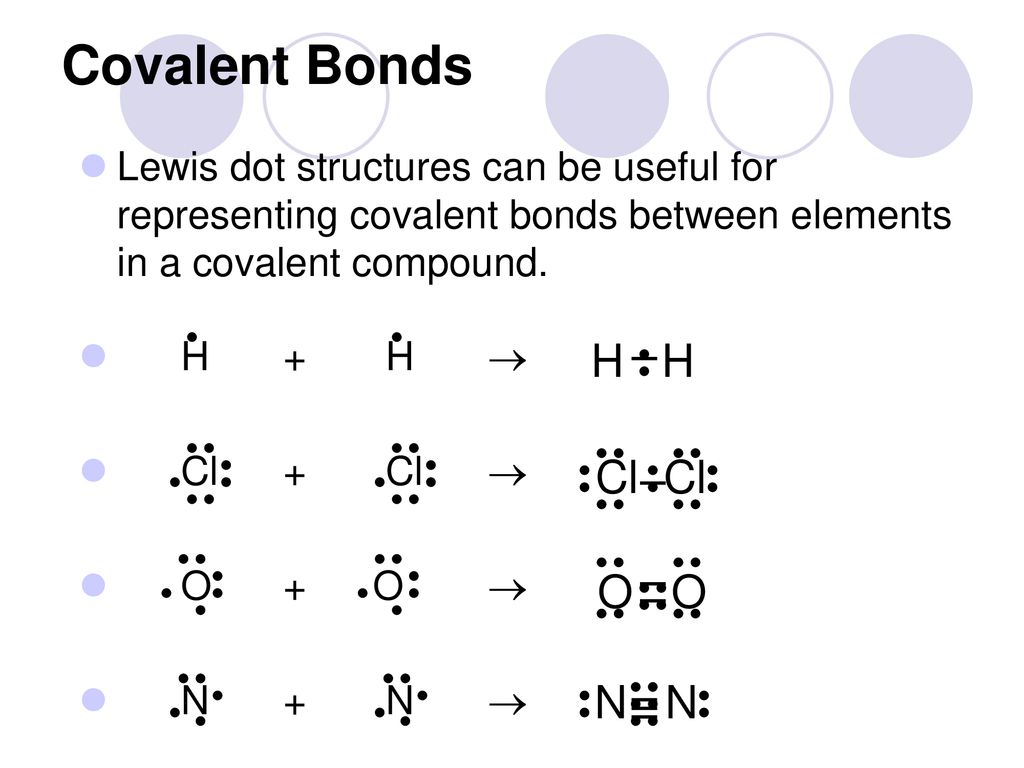

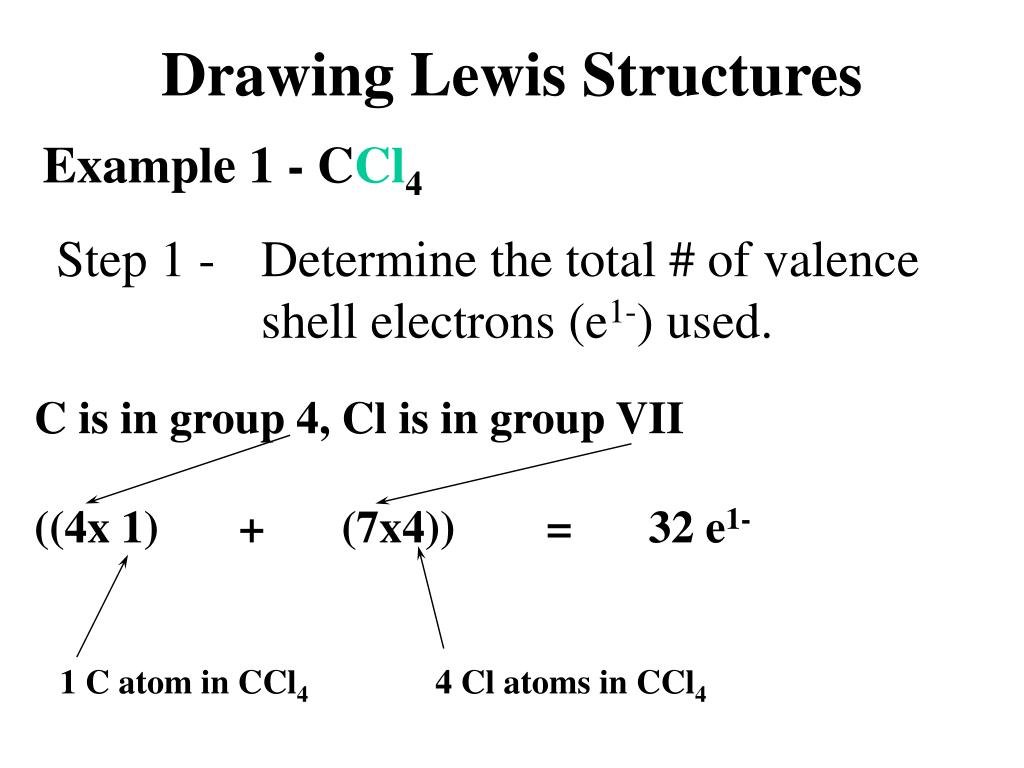

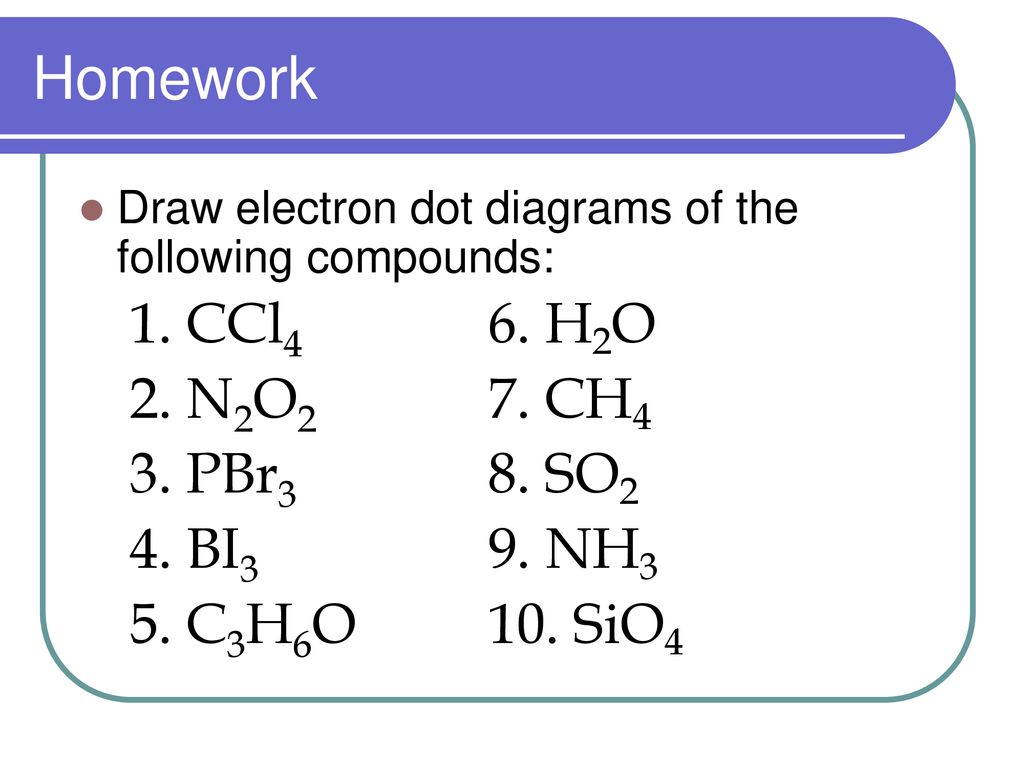





0 Response to "40 lewis dot diagram for ccl4"
Post a Comment