40 lewis diagram for ccl4
In the Lewis structures listed here, M and X represent various elements in the third period of the periodic table. Write the formula of each compound using the chemical symbols of each element: (a) (b) (c) (d) Write the Lewis structure for the diatomic molecule P 2, an unstable form of phosphorus found in high-temperature phosphorus vapor. The completed Lewis structure for CCl4 is shown in the following figure: Step 7. Check to see if any of the atoms in the molecule have a positive or negative charge. Add the number of lone pair electrons to the number of bonds for each atom in the molecule. Subtract this number from the number of valence electrons the atom is expected to have ...
A CCL4 Lewis structure is a diagram that represents the electron setup of covalently bonded substances. Therefore, a carbon atom will share each of its 4 external electrons with a single chlorine atom, offering the single carbon atoms and 4 chlorine atoms a complete external shell of electrons. …

Lewis diagram for ccl4
Lewis Dot Structure and Polarity of CCl4 (Carbon Tetrachloride) Carbon tetrachloride, also known as tetrachloromethane, is a compound containing carbon and chlorine. It is an inorganic compound that is non-flammable. This ScienceStruck post provides you with the Lewis dot structure diagram and the polarity of carbon tetrachloride. CCl4 lewis's structure is made up of one carbon atom that is situated at the middle position and four chlorine atoms that are at the surrounding position. The total lone pair present in the CCl4 lewis dot structure is 12. Lone pairs of electrons do not involve in chemical bonds and it is represented as a dot in the lewis diagram. According to the Lewis structure, CCl4 is a tetrahedral molecule. With this molecule there is no way to draw a line to separate the charges. In fact, since the molecule is symmetrical, all the dipole moments will cancel each other out.CCl4 is an example of a nonpolar molecule.
Lewis diagram for ccl4. I quickly take you through how to draw the Lewis Structure of CCl4 (Carbon TetraChloride). I also go over hybridization, shape and bond angle. What Is The Lewis Structure For Ccl4. ccl4 is also called Carbon Tetachloride. We called it corbon Tetachloride because C mean corbon and cl mean chloride and 4 call as teta in chemistry so it is called corbon Tetachloride.. The levis structure is diagram That Represent That Configuration Of Electron Of Covalently Bonded Compounds. A CCL4 Lewis structure is a diagram that represents the electron configuration of covalently bonded compounds.Lewis structures are meant to provide a visualization of the atomic structure and the distribution of electrons in a given chemical compound.. Carbon tetrachloride (CCl 4) is a covalently bonded compound composed of a central carbon surrounded by 4 chlorine atoms in a tetrahedral ... A CCL4 Lewis structure is a diagram that represents the electron configuration of covalently bonded compounds. Lewis structures are meant to provide a visualization of the atomic structure and the distribution of electrons in a given chemical compound. A regular atom of carbon has 4 lone electrons in its outer shell.
A CCL4 Lewis structure is a diagram that represents the electron configuration of covalently bonded compounds. Lewis structures are meant to provide a visualization of the atomic structure and the distribution of electrons in a given chemical compound. A regular atom of carbon has 4 lone electrons in its outer shell. 1) Draw the Lewis Structure of CCl4. What is the electron geometry of the central C atom? What is the molecular geometry of the central C atom? What is the hybridization of the central C atom? What is the molecular geometry or shape of the molecule? Does CCl4 have a dipole moment? 2) Draw the Lewis Structure of H2S. Ccl4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram. Carbon Tetrachloride is a chemical that is produced and is not available naturally. In its natural state, it is a colorless liquid chemical with a little sweet smell like ether. Which of the following molecules or ions will have a Lewis structure most like that of phosphorus trichloride, PCl3? ClO3-Which of the following is/are possible Lewis structures for C2H6O? ... CCl4, Cl2, HCl, and KCl? CCl4 and HCl. Which of the following molecules are polar: H2S, CO2, NH3, BH3, and CCl4?
CCl4 Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram Carbon Tetrachloride is a chemical that is produced and is not available naturally. In its natural state, it is a colorless liquid chemical with a little sweet smell like ether. CF4 lewis structure contains one carbon and four fluorine atom, carbon is in the center, and all fluorine atoms surrounding it. No lone pair present on the central atom of the CF4 lewis dot structure but 3 lone pairs present on each outer atom. The lewis diagram of CF4 is very similar to CCl4. Let's see step by step how to draw this in a very ... Draw the Lewis structure for the CCl4 Select the correct hybridization for the central atom based on the electron geometry CCl4 Draw the Lewis structure for the NH3. Select the correct hybridization for the central atom based on the electron geometry NH3. Draw the Lewis structure for the OF2. A step-by-step explanation of how to draw the CCl4 Lewis Dot Structure (Carbon tetrachloride). The Lewis structure for CCl4 is a commonly tested Lewis struc...
Lewis diagram for ccl4 Lewis dot diagram for ccl4. Hi, today I'm going to design the CCl4 Lewis structure for CCl4 in just four steps. CCl4 Lewis Structure Setup Step-1: To design the CCl4 Lewis structure (carbon tetrachloride), we must first discover the electrons of CC4 valence.

Slovak Steel Worker in the Pittsburgh Region Relaxes after Supper (1909) // Lewis Wickes Hine American, 1874–1940
Let's do the Lewis structure for CCl4, Carbon Tetrachloride, sometimes just called Carbon Tet. We'll start by looking at the valence electrons. Carbon is in group 4 or 14, so it has 4. Chlorine has 7 valence electrons, but we have 4 Chlorines so let's multiply that by 4. Four plus 28 equals 32 total valence electrons to work with.
2. Draw the Lewis dot structures for each of the following molecules: a. H 2 S c. SO 3 b. CH 2 Br 2 d. HCN 3. Draw the Lewis dot structure for each of the following polyatomic ions: a. NH 4 + c. PO 4 -3 b. NO 3 - d. CO 3 2- 4. For the following molecules or ions (where the central atom is underlined): i. Draw the Electron dot structure. ii.
Answer (1 of 4): The free electron pairs repulse the other substituents. The XY4 molecules are always more symmetric than :XY3 molecules. That is true is case of octahedral geometry: XY6 vs. :XY5.
Sticks or straight lines represent the bonds. While the dots represent the non-bonding electrons. Lewis theory is based on the octet rule, which states that an atom should have eight electrons in its outer shell to be stable. For the Lewis structure of CCl4 first, let's calculate the total valence electrons.
Lewis Structures. What are the Lewis structures of various compounds? 1. What is the Lewis structure for Water? 2. 3. What is the Lewis formula for ammonia (NH 3 )? 4.
All the non-bonding electrons in CCl4 tends to create a repulsive force among each other resulting in the bond angle of 109.5 degrees with each other and resulting in a tetrahedral shape. For detailed information about valence electrons and the lewis structure, you must go through the lewis structure, hybridization of CCl4. Synthesis of CCl4
This problem has been solved! Draw the Lewis structure of CCl4. Include all the lone pairs. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. Transcribed image text: Draw the Lewis structure of CC14.

Margaret Frances Langton Clarke (1864, printed c. 1866) // Lewis Carroll (Reverend Charles Lutwidge Dodgson) English, 1832–1898
Lewis structure of ClO 3-ion Lewis structure of ClF 3 Lewis structure of water Lewis structure of SO 3 2-M N NO Lewis tructure NO 2 Lewis tructure NH 3 Lewis tructure NF 3 Lewis tructure NOCl Lewis structure O OCl 2 Lewis structure P Predict polarity of molecules PH 3 Lewis structure PCl 5 Lewis structure Q R: S SF6 Lewis structure SiF 4 lewis ...
Carbon tetrachloride is a manufactured chemical that does not occur naturally. It is a clear liquid with a sweet smell that can be detected at low levels. It is also called carbon chloride, methane tetrachloride, perchloromethane, tetrachloroethane, or benziform.Carbon tetrachloride is most often found in the air as a colorless gas.
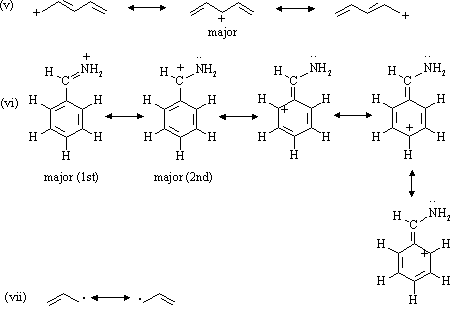
In this example, we can draw two Lewis structures that are energetically equivalent to each other — that is, they have the same types of bonds, and the same types of formal charges on all of the structures.Both structures (2 and 3) must be used to represent the molecule's structure.The actual molecule is an average of structures 2 and 3, which are called resonance structures.
According to the Lewis structure, CCl4 is a tetrahedral molecule. With this molecule there is no way to draw a line to separate the charges. In fact, since the molecule is symmetrical, all the dipole moments will cancel each other out.CCl4 is an example of a nonpolar molecule.
CCl4 lewis's structure is made up of one carbon atom that is situated at the middle position and four chlorine atoms that are at the surrounding position. The total lone pair present in the CCl4 lewis dot structure is 12. Lone pairs of electrons do not involve in chemical bonds and it is represented as a dot in the lewis diagram.
Lewis Dot Structure and Polarity of CCl4 (Carbon Tetrachloride) Carbon tetrachloride, also known as tetrachloromethane, is a compound containing carbon and chlorine. It is an inorganic compound that is non-flammable. This ScienceStruck post provides you with the Lewis dot structure diagram and the polarity of carbon tetrachloride.

Sadie Pfeifer, a Cotton Mill Spinner, Lancaster, South Carolina (1908) // Lewis Wickes Hine American, 1874–1940






















0 Response to "40 lewis diagram for ccl4"
Post a Comment