40 energy level diagram chemistry
Important observations from energy level diagrams of multi electron atoms are: 1)The sub shell of a particular shell do not have equal energies.For Ex: 2s and 2p have different energies. 2)In a particular shell, sub shell with lower value of l has lower energy. In the second shell, 2s ( l=0) has lower energy than 2p ( l=1) .In the 3rd shell ... 7:33In this video, I will teach you how to draw energy level diagrams, which is a better way of visualizing how ...17 Feb 2013 · Uploaded by ThatSingaporeanGuy
Chemists sometimes use an energy level diagram to represent electrons when they're looking at chemical reactions and bonding. An energy level diagram is ...
Energy level diagram chemistry
The energy level diagram is therefore as shown below. Note that the total pi electron energy is more negative (more bonding) than the two electrons of the ethene pi bond, by 0.82b. This is the delocalization energy, i.e., the additional stabilization of these two electrons derived from their delocalization over three atoms, instead of just two. Energy level diagrams. are used to model energy. changes during reactions. They show the relative energy levels of the products. and reactants . 9 Jun 2020 — Typically, at low energies, the levels are far apart. At higher energies, they are closer together. At sufficiently high energy, an electron is ...
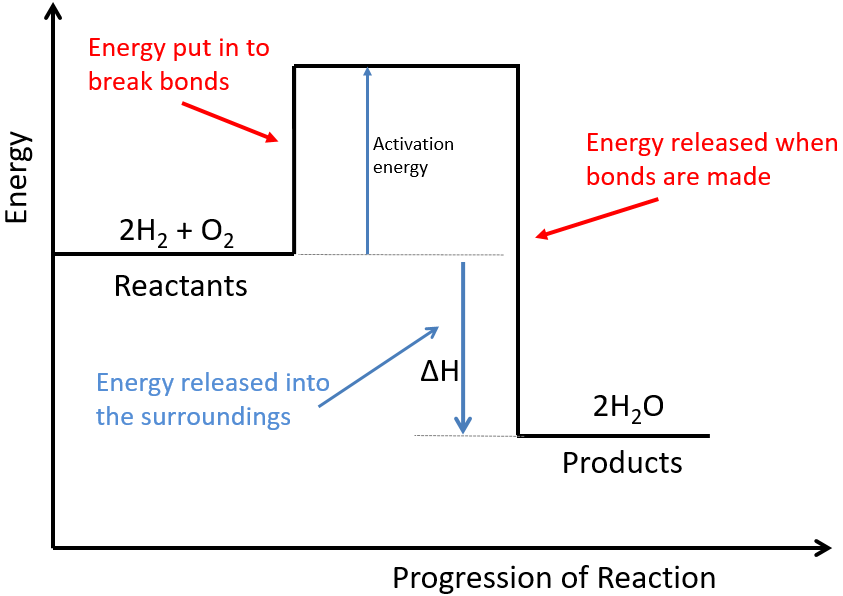
Energy level diagram chemistry. Worked example: Drawing energy level diagrams of the combustion of methane. Step 1: The chemical equation for the complete combustion of methane is: Step 2: Combustion reactions are always exothermic (Δ H is negative) so the reactants should be drawn higher in energy than the products. Step 4: Draw arrows to show the Ea and Δ H including ... 2:30Chemistry Reading list... https://www.primrosekitten.com/collec... Some of the links in here are affiliate links ...3 Apr 2015 · Uploaded by Science and Maths by Primrose Kitten What is energy level diagram? In chemistry, an electron shell, or energy level, may be imagined as an orbit with electrons around the nucleus of an atom. The closest shell to the nucleus is called the “K shell” followed by the “L shell” then the “M shell” and so on away from the nucleus. The shells can be denoted by alphabets (K, L ... Worked example: Drawing energy level diagrams of the combustion of methane. Answer. Step 1: The chemical equation for the complete combustion of methane is: CH4 (g) + 2O2 (g) → CO2 (g) + 2H2O (l) Step 2: Combustion reactions are always exothermic (Δ H is negative) so the reactants should be drawn higher in energy than the products.
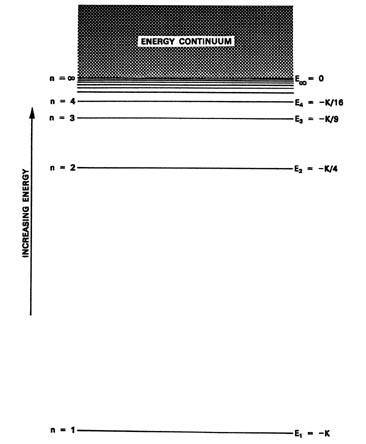
Filed Under: Chemical Bonding and Molecular Structure, Chemistry, Class 11 Tagged With: bond order, Energy level diagram for Molecular orbitals About Mrs Shilpi Nagpal Author of this website, Mrs Shilpi Nagpal is MSc (Hons, Chemistry) and BSc (Hons, Chemistry) from Delhi University, B.Ed (I. P. University) and has many years of experience in ... Enthalpy level diagrams. When a Hess' law energy cycle is presented graphically showing the relative chemical energies of the reactants and products, this is called an energy, or enthalpy level diagram. Personally, I find these more logical than Hess' energy cycles, however it's a matter of individual preference. The energy spectrum of a system with such discrete energy levels is said to be quantized. In chemistry and atomic physics, an electron shell, or principal ... Chemistry Lesson 3.2Energy Level DiagramsPauli Exclusion PrincipleHund's RuleAufbau PrincipleDiagonal RulePractice problems#energyLevelDiagram #quantum #ketz...
An energy level diagram is a diagram that shows the energies of the reactants, the transition state(s) and the products of the reaction with time; The transition state is a stage during the reaction at which chemical bonds are partially broken and formed Chemistry 2017. DAY ONE: October 3 ... •In each energy level, electrons fill sublevels in a certain order Level 1: a) only has one s sublevel (a spherical shape) ... diagram. Order of Orbitals—Periodic Table For the d block n-1 For the f block n-2. Hotel analogy video s s s s p d d p p f These are 4 levels each 7:49Chemistry Lesson - 12 - Energy Level Diagram and Electron Configuration. 111,107 views111K views. Oct 22 ...22 Oct 2011 · Uploaded by thenewboston 18:26Bohr Model of the Hydrogen Atom, Electron Transitions, Atomic Energy Levels, Lyman & Balmer Series. The ...16 Sep 2014 · Uploaded by Mindset
9 Jun 2020 — Typically, at low energies, the levels are far apart. At higher energies, they are closer together. At sufficiently high energy, an electron is ...
Igcse Chemistry 2017 3 13 Know That A Catalyst Works By Providing An Alternative Pathway With Lower Activation Energy
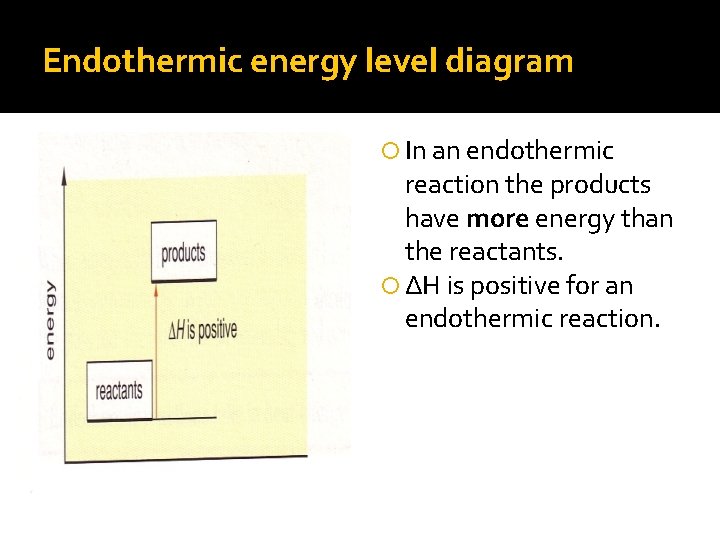
Energy level diagrams. are used to model energy. changes during reactions. They show the relative energy levels of the products. and reactants .
The energy level diagram is therefore as shown below. Note that the total pi electron energy is more negative (more bonding) than the two electrons of the ethene pi bond, by 0.82b. This is the delocalization energy, i.e., the additional stabilization of these two electrons derived from their delocalization over three atoms, instead of just two.
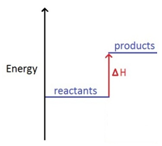
3 05 Triple Only Draw And Explain Energy Level Diagrams To Represent Exothermic And Endothermic Reactions Tutormyself Chemistry
Igcse Chemistry 4 14 Represent Exothermic And Endothermic Reactions On A Simple Energy Level Diagram
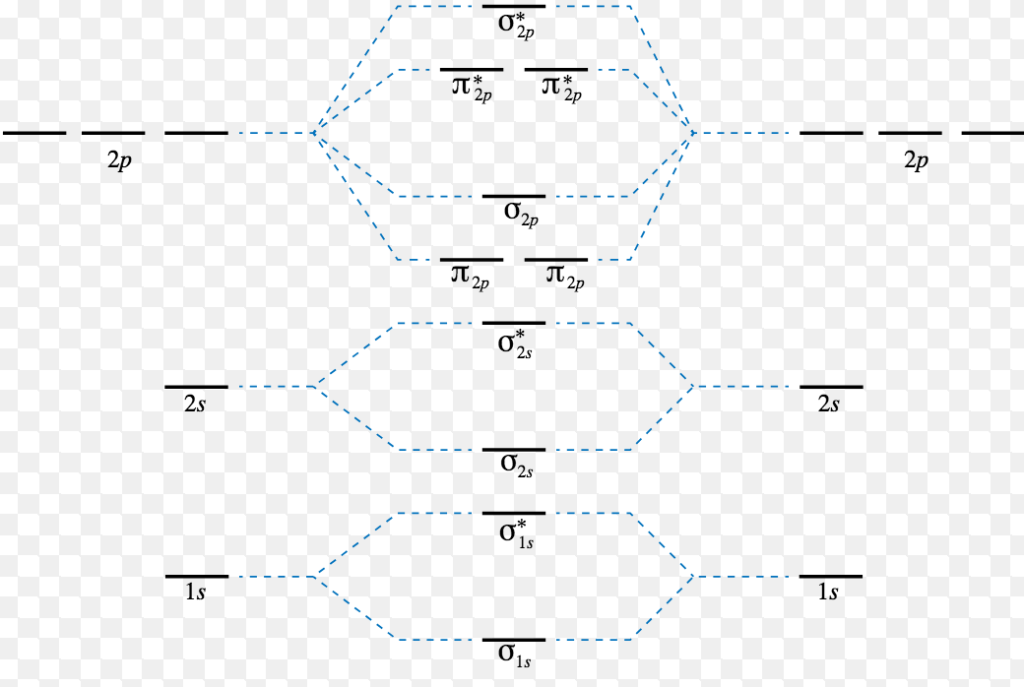
Energy Level Diagram For Molecular Orbitals Chemical Bonding And Molecular Structure Chemistry Class 11
Igcse Chemistry 2017 3 14c Draw And Explain Reaction Profile Diagrams Showing H And Activation Energy
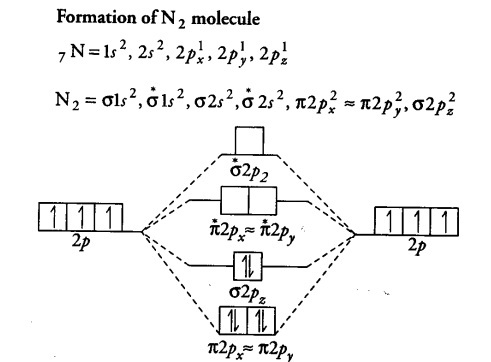
Use The Molecular Orbital Energy Level Diagram To Show That Cbse Class 11 Chemistry Learn Cbse Forum





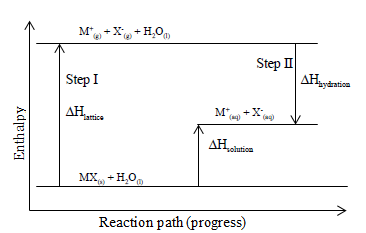




















0 Response to "40 energy level diagram chemistry"
Post a Comment