39 orbital diagram for lithium
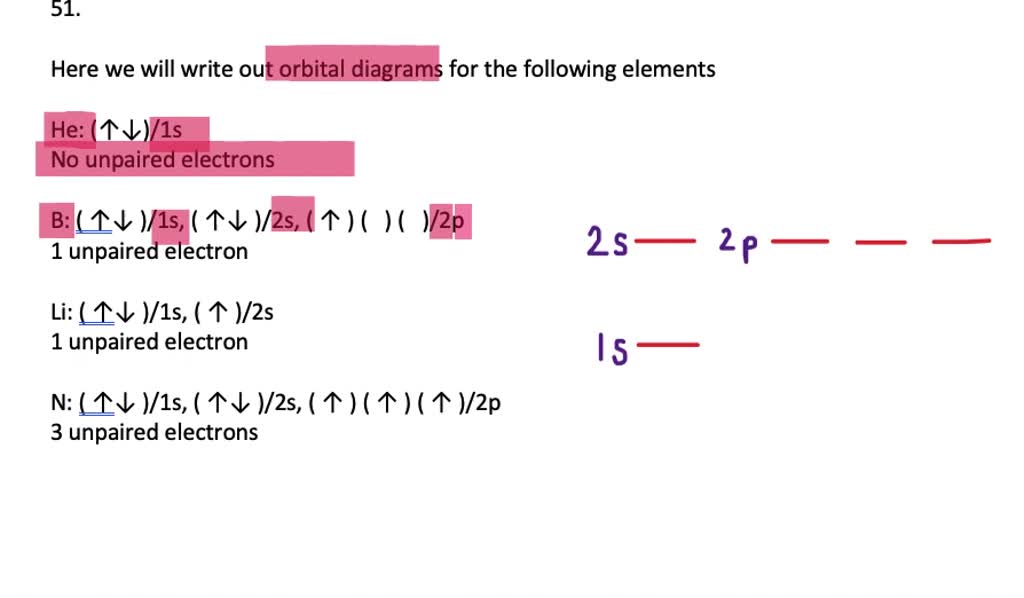
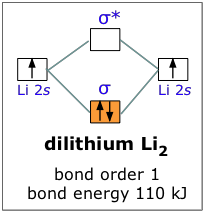
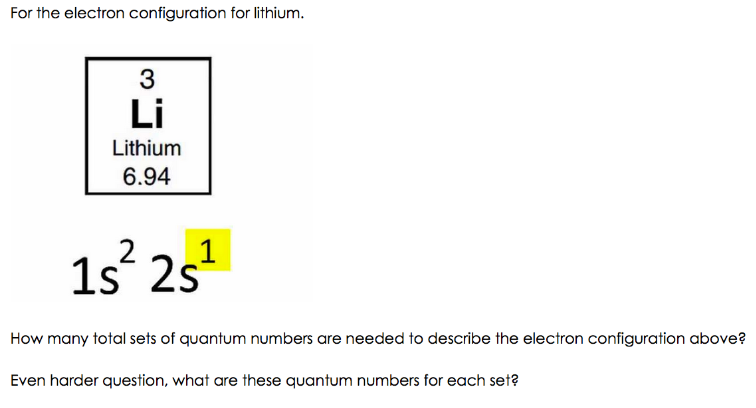
Science; Chemistry; Chemistry questions and answers; Electron Configurations Worksheet Write the complete ground state electron configurations and orbital notations for the following: #of e Element (atom) e configuration Orbital Notations/ diagrams 1) 3 lithium 15^2 2541 [1L] [1] 2) 8 oxygen 18^2 25^2 2P44 [1L] [1L] [11][1][1] [11] [11] [14][14][14] [11] [14][14][14] [1L] 3) 20 calcium 182 25 ... Molecular Orbital Diagram of H2 Molecule: Molecular Orbital Theory for Homonuclear Diatomic . Molecules Lithium molecule (Li2): The formation of Lithium (Li) molecule is made possible by the overlapping of two lithium atoms and each consisting of electronic configuration 1s2 2s1.
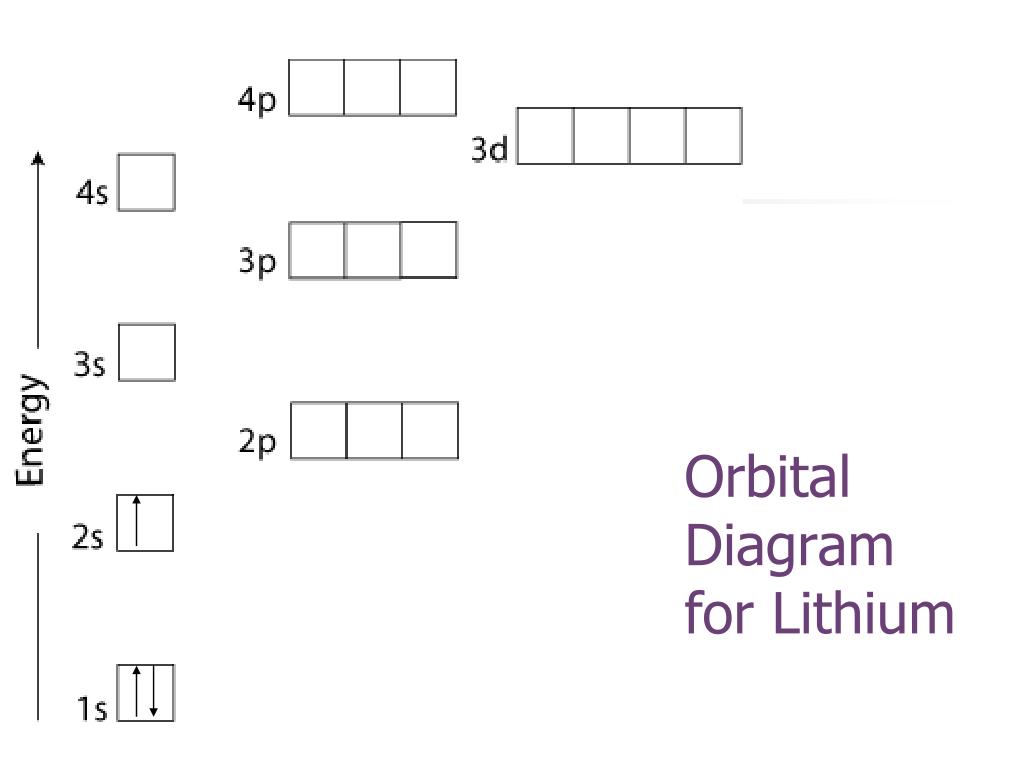
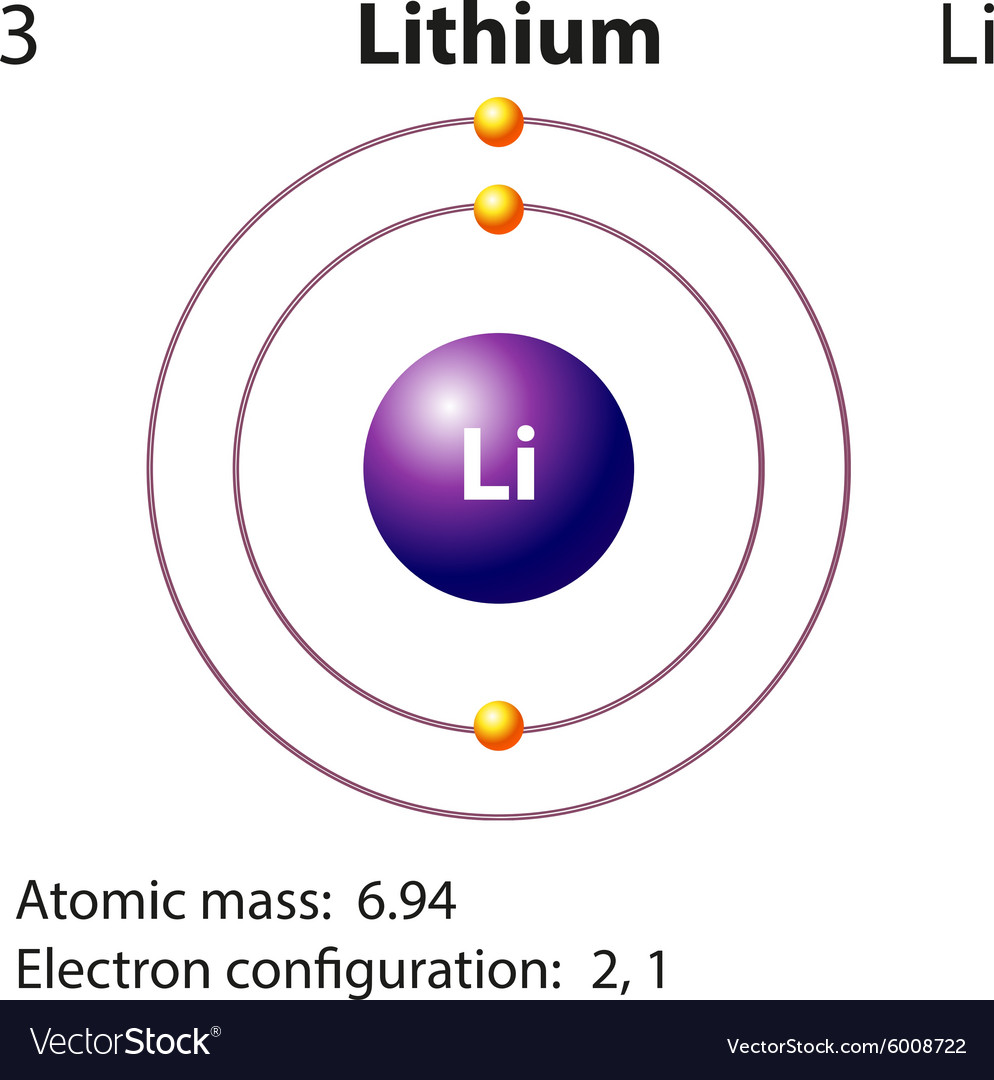
For example, the 5s orbital is of lower energy than the 4d orbital (see Figure 3 for a complete pattern of orbital levels). Ca electron occupancy The orbital notation of calcium (Ca) is 1s2 2s2 2p6 3s2 3p6 4s2 Or short-hand: [Ar] 4s2. The orbital filling diagram of lithium. The electron configuration of lithium is 1s²2s¹.

Orbital diagram for lithium
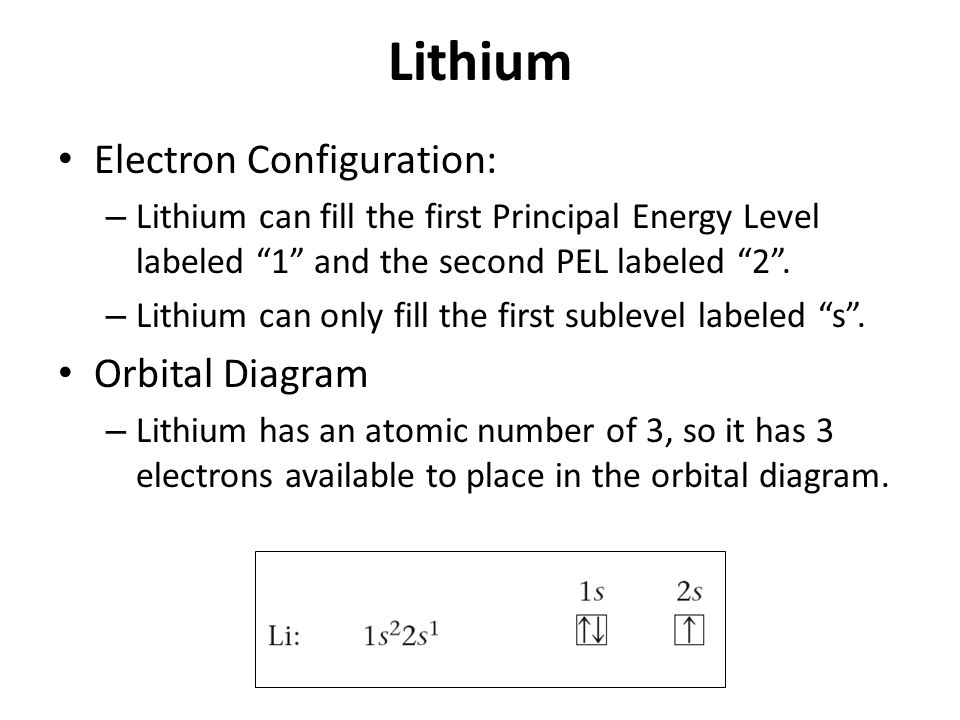
The lithium 1s orbital is the lowest-energy orbital on the diagram. Because this orbital is so small and retains its electrons so tightly, it does not contribute to bonding; we need consider only the 2 s orbital of lithium which combines with the 1 s orbital of hydrogen to form the usual pair of sigma bonding and antibonding orbitals. Hint: The number of molecular orbitals formed is equal to the number of interacting atomic orbitals. You can use the following formula for the bond order. \[BO = \dfrac{{{N_b} - {N_a}}}{2}\] Here, \[BO,{N_b}\] and \[{N_a}\] represents the bond order, the number of electrons in bonding molecular orbitals and the number of electrons in antibonding molecular orbitals respectively. The first two electrons in lithium fill the 1s orbital and have the same sets of four quantum numbers as the two electrons in helium. The remaining electron must occupy the orbital of next lowest energy, the 2s orbital (Figure 3 or Figure 4). Thus, the electron configuration and orbital diagram of lithium are:
Orbital diagram for lithium. Neon electron configuration is 1s 2 2s 2 2p 6.The symbol for neon is 'Ne' and it is an inert element. This article gives an idea about the electron configuration of neon(Ne) and orbital diagram, period and groups, valency and valence electrons of neon, application of different principles. The tenth element in the periodic table is neon. Lithium is the third element with a total of 3 electrons. In writing the electron configuration for lithium the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the remaining electron for Li goes in the 2s orbital. Draw the M.O diagram for oxygen molecule and calculate its bond order and show that O2 is paramagnetic. asked Dec 17, 2020 in Chemical Bonding by Panna01 ( 47.2k points) chemical bonding The first two electrons in lithium fill the 1s orbital and have the same sets of four quantum numbers as the two electrons in helium. The remaining electron must occupy the orbital of next lowest energy, the 2s orbital (Figure 6.26 or Figure 6.27). Thus, the electron configuration and orbital diagram of lithium are:
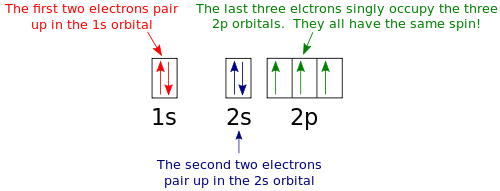
An orbital box diagram can be written as well. Boxes, or horizontal lines represent the orbitals, arrows represent the electrons, and if an orbital is full, the electrons must be of opposite spin-one arrow pointing up and the other one pointing down. The orbital box diagrams are listed for the first 20 elements in the figure below. The first two electrons in lithium fill the 1s orbital and have the same sets of four quantum numbers as the two electrons in helium. The remaining electron must occupy the orbital of next lowest energy, the 2s orbital (Figure 3 or Figure 4). Thus, the electron configuration and orbital diagram of lithium are: What is the orbital notation for lithium? ... Learn the definition of electron and electron cloud, learn about the electron cloud model, and view an electron cloud diagram. The diagram shows how the molecular orbitals in lithium hydride can be related to the atomic orbitals of the parent atoms. O2 Molecular Orbital Diagram Oxygen has a similar setup to H 2, but now we consider 2s and 2p orbitals.
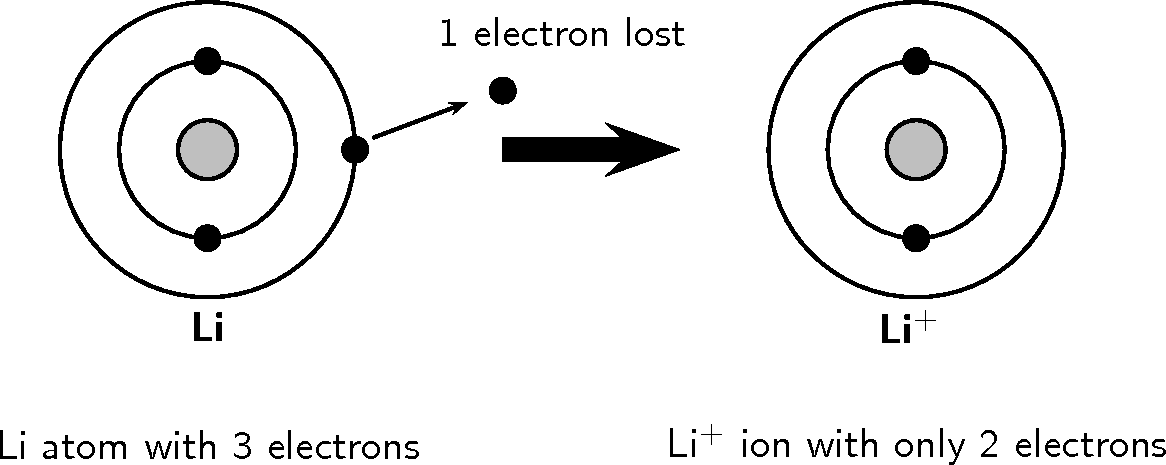
This means that a neutral lithium atom will have a total of 3 electrons surrounding its nucleus. Its electron configuration will be. Li: 1s22s1. Now, the lithium cation, Li+, is formed when lithium loses the electron located on its outermost shell → its valence electron. This electron is located on the second energy level, in the 2s-orbital. A molecular orbital can hold two electrons, so both electrons in the H 2 molecule are in the [latex]\sigma[/latex] 1s bonding orbital; the electron configuration is [latex]{\left({\sigma}_{1s}\right)}^{2}.[/latex] We represent this configuration by a molecular orbital energy diagram (Figure 7.7.10) in which a single upward arrow indicates one ... Lithium atomic orbital and chemical bonding information. There are also tutorials on the first thirty-six elements of the periodic table. Check out the blackboard. That box on the left has all of the information you need to know about one element. It tells you the mass of one atom, how many ... It can be stated the 2s orbital penetrates closer to the nucleus than does a 2p orbital. So the orbital diagram for lithium is shown below. The electron configuration for lithium is 1s 2 2s 1. The energy level diagram, on the left shows the relative energy of the 2s and 2p orbitals based on the ability of the sublevels to penetrate to the ...
Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics.
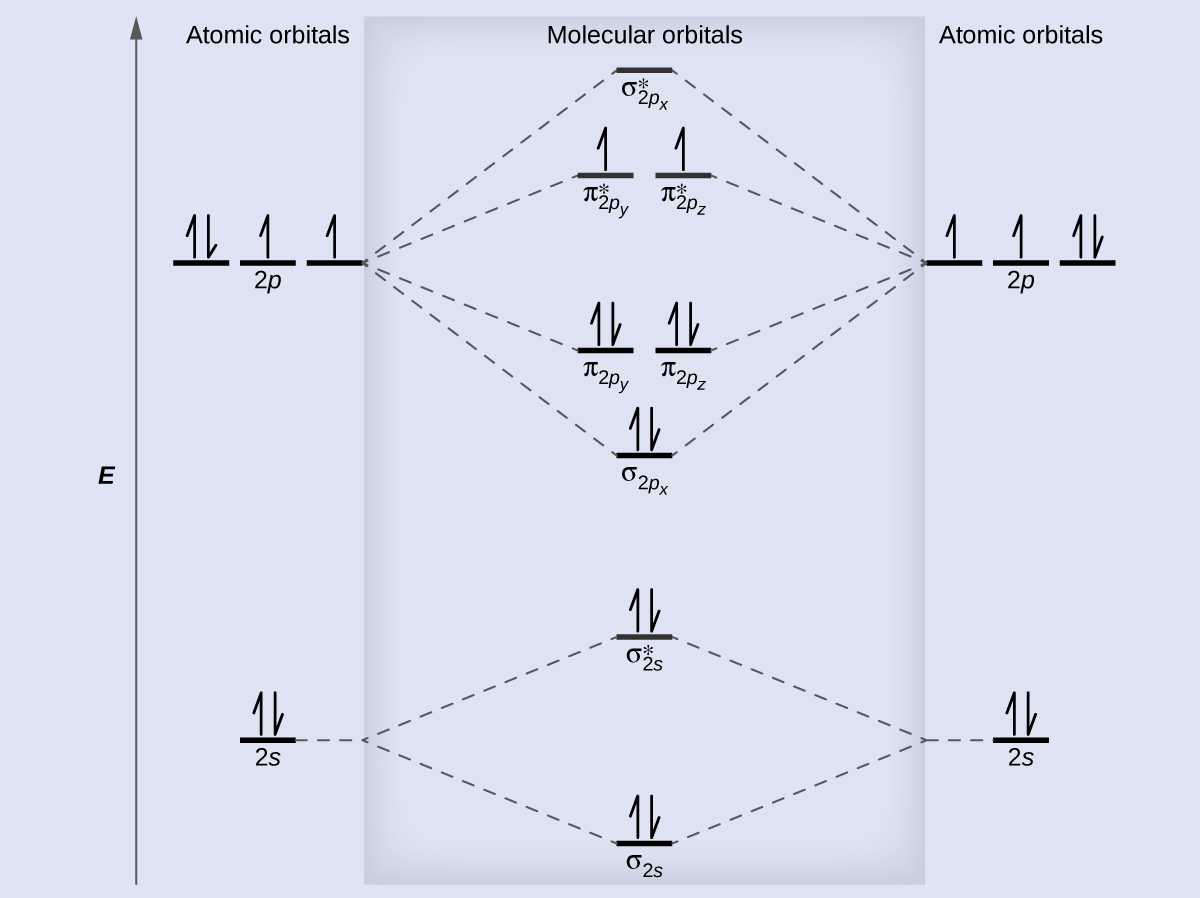
According to the molecular orbital theory ,the molecular orbital diagram of lithium molecule is shown below: The 2 electrons are present in σ 2s orbitals . The anti bonding molecular orbital σ*2 s is empty . Thus . Bond order = Number of electrons in BMO ...
Since the 1s orbital is of lower energy than the 2s orbital, the 1s orbital should be filled first, and any remaining electron should be used to fill the 2s orbital, making configuration A the correct orbital diagram for Lithium.
The orbital filling diagram of lithium. The electron configuration of lithium is 1s²2s¹. This means that there are two electrons in the 1s orbital, and one electron in the higher energy 2s orbital. If you want to make a cool picture, you can do it like this:
Bohr diagram of beryllium in addition bohr diagram of magnesium oxide furthermore n15 atom bohr diagram also diagram architecture plan stock photo along with pare argon radon furthermore electronic structure theories furthermore key diagram of power station also pare arsenic silicon together with drawing of lithium bohr diagram for 3 also.
Nov 01, 2021 · Orbital Diagram of All Elements Diagrams; 1: Orbital diagram of Hydrogen (H) 2: Orbital diagram of Helium (He) 3: Orbital diagram of Lithium (Li) 4: Orbital diagram of Beryllium (Be) 5: Orbital diagram of Boron (B) 6: Orbital diagram of Carbon (C) 7: Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine ...
Orbital diagram for lithium. the atomic formula of lithium is 3 . so the electronic concentration will be 2,1 . this means that there will be 2 electrons in first shell n 1 electron in second shell.
Lithium electron configuration and orbital diagram are the main topics of this article. Also, period and group determination, valency and valence electrons, various reactions and compound formation of lithium, bond formation of lithium have been discussed.
Orbital diagram for lithium? the atomic formula of lithium is 3 . so the electronic concentration will be 2,1 . this means that there will be 2 electrons in first shell n 1 electron in second shell.
The first two electrons in lithium fill the 1s orbital and have the same sets of four quantum numbers as the two electrons in helium. The remaining electron must occupy the orbital of next lowest energy, the 2s orbital (Figure 3 or Figure 4). Thus, the electron configuration and orbital diagram of lithium are:

How To Draw Molecular Orbital Diagram Of Li2 Li 2 Li2 Simplest Trick Chemistry Best Online Free Chemistry Class 9 12
Molecular Orbital Diagram of Lithium Molecule Video Lecture from Chapter Nature of Chemical Bond of Subject Chemistry Class 11 for HSC, IIT JEE, CBSE & NEET....
The illustration above uses the hydrogen wavefunctions, which are not exactly correct for lithium but can be used to obtain a qualitative understanding of the dependence of the electron energies on the orbital quantum number. If there were no shielding of the 2s electron, it would be exposed to the entire nuclear charge and have energy -30.6 eV.
Orbital filling diagrams for hydrogen, helium, and lithium. According to the Aufbau process, sublevels and orbitals are filled with electrons in order of increasing energy. Since the s sublevel consists of just one orbital, the second electron simply pairs up with the first electron as in helium.
The first two electrons in lithium fill the 1s orbital and have the same sets of four quantum numbers as the two electrons in helium. The remaining electron must occupy the orbital of next lowest energy, the 2s orbital (Figure 3 or Figure 4). Thus, the electron configuration and orbital diagram of lithium are:
Hint: The number of molecular orbitals formed is equal to the number of interacting atomic orbitals. You can use the following formula for the bond order. \[BO = \dfrac{{{N_b} - {N_a}}}{2}\] Here, \[BO,{N_b}\] and \[{N_a}\] represents the bond order, the number of electrons in bonding molecular orbitals and the number of electrons in antibonding molecular orbitals respectively.
The lithium 1s orbital is the lowest-energy orbital on the diagram. Because this orbital is so small and retains its electrons so tightly, it does not contribute to bonding; we need consider only the 2 s orbital of lithium which combines with the 1 s orbital of hydrogen to form the usual pair of sigma bonding and antibonding orbitals.









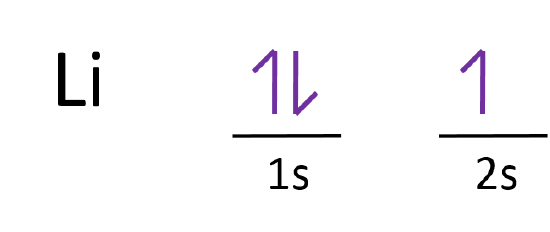


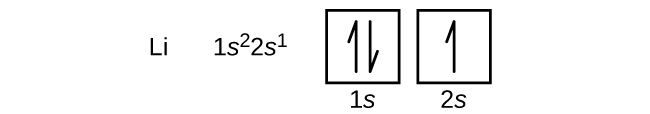



0 Response to "39 orbital diagram for lithium"
Post a Comment