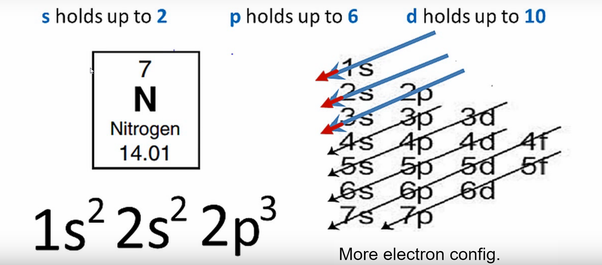
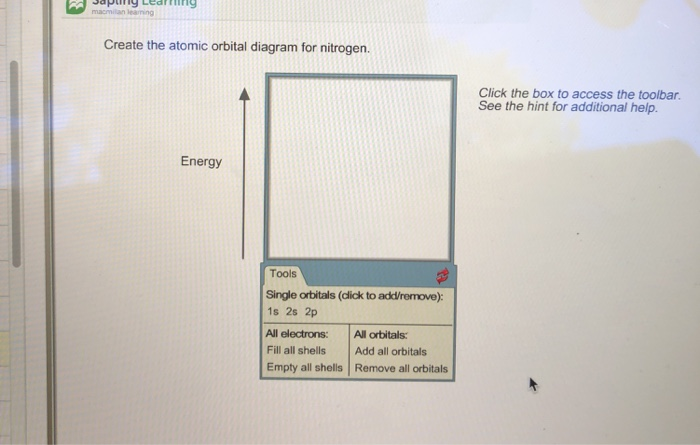
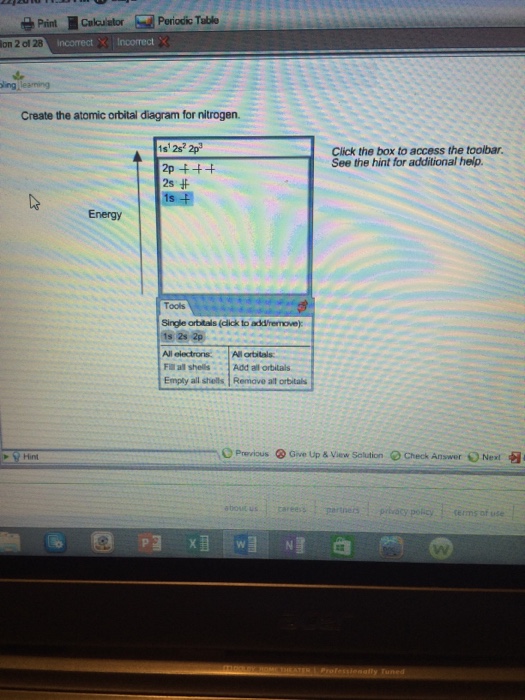
38 create the atomic orbital diagram for nitrogen.
Nitrogen (N) has an atomic mass of 7. Find out about its chemical and physical properties, states, energy, electrons, oxidation and more. The Electron Configuration. Q. The innermost electron shell of an atom can hold up to _ electrons. Solved • Mar 17, 2021. The Electron Configuration. Q. The element that corresponds to the electron configuration 1s22s22p63s23p64s13d5 is: A) titanium B) vanadiumC) mangneseD) chromium. Solved • Jan 27, 2021.
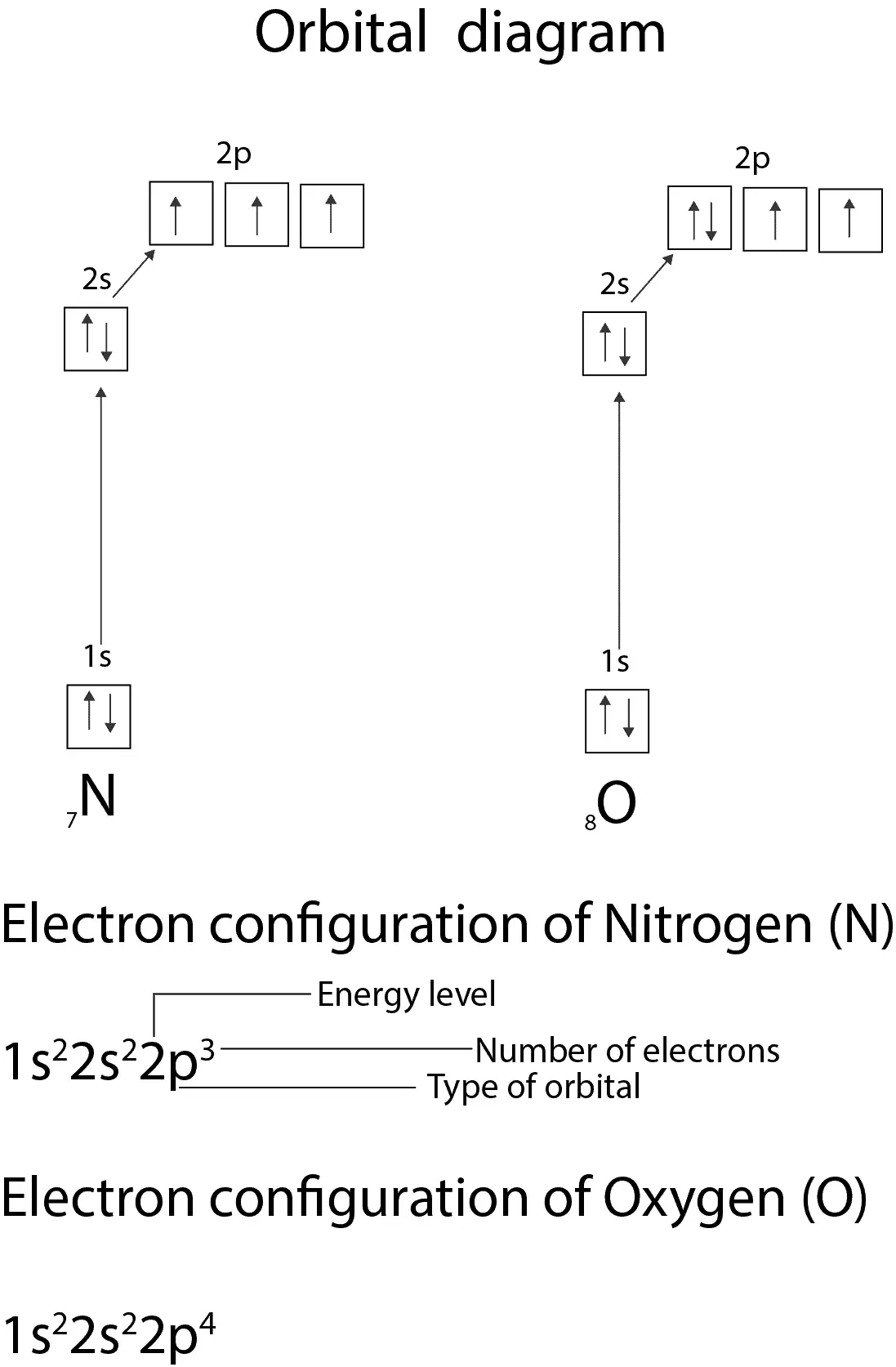
Figure 6.29 tells us that the next lowest power orbital is 2 s, therefore the orbital diagram for lithium is. with 3 unpaired electrons. The electron configuration of nitrogen is therefore 1 s 22 s 22 p 3. At oxygen, v Z = 8 and also eight electrons, we have actually no choice.

Create the atomic orbital diagram for nitrogen.
Nitrogen atomic number 7 fills the 1 s and 2 s subshells and has one electron in each of the three 2 p orbitals in accordance with Hunds rule. The number of isotopes possible b. Step 1 Find the symbol for the element on a periodic table. NF3 Lewis Structure, Molecular Geometry, Hybridization, Polarity, and MO Diagram. Nitrogen trifluoride or NF3 is a nitrogen halide compound that is slightly water-soluble. Its noticeable characteristics include being colorless and carrying a musty or moldy odor. NF3 has a molar mass of around 71.002 g/mol and a density of 3.003 kg/m3. Atomic number, atomic mass, protons, neutrons, electrons. Dřañ a bohr model of an oxygen atom in the space below. Label them with their charge. Determine the number of electrons in each of the atomic diagrams in model 1. "provide an orbital energy level diagram for the ground state of a nitrogen atom." in .
Create the atomic orbital diagram for nitrogen.. FREE Answer to 1. Create the atomic orbital of diagram for nitrogen. 2. Construct the orbital diagram for Ni. So here, we need to create an atomic orbital diagram for nitrogen. Let's go ahead and write its electron configuration first, though, ... Create the atomic orbital diagram for chlorine. Our videos will help you understand concepts solve your homework and do great on your exams. Create the atomic orbital diagram for chlorine. The las o is supposed. Phosphorus 1s 2s 2p 3s 3p 4s 3d 4p. This video tutorial shows you how to fill the orbital diagrams using boxes and arrows plus how to ... In order to continue enjoying our site, we ask that you confirm your identity as a human. Thank you very much for your cooperation
For example, aluminum has the atomic number 13, which is the number of protons in the nuclei of its atoms. It is written out, as opposed to orbital diagrams which are depicted pictorially. Write the shorthand electron configuration for each of the following. Oganesson (element 118 is a good example to show the order of the orbitals. How to Write Electron Configurations. Step-by-step tutorial for writing the Electron Configurations. Create the atomic orbital diagram for nitrogen. Start by adding the appropriate subshells. For example, boron is in the 2p block of the periodic table, and so ... November 19, 2016 - Answer: No random, high-energy and free electrons. Maximum electrons in first two orbitals: 10. 7 electrons are present. Electrons are therefeore limited to two orbitals. First orbital: 1s2 Second orbital: 2s2 2p3 Combined electron configuration: 1s2 2s2 2p3 Draw two electrons for the firs...
Electron Orbitals: Electron Configuration Orbital Diagram Worksheet Answers (The electron configuration orbital diagram worksheet answers can be found at the bottom of the lesson.) The 2, 8, 8, 18 rule is a very simplistic view of electron configuration and doesn't give the full picture when it comes to electron configuration. The figure below illustrating orbital diagrams for nitrogen is similar to the orbital diagram for carbon in the first figure. Notice how all three 2p electrons in the orbital diagram on the left are in separate orbitals, while two of the three 2p electrons in the diagram on the right are sharing ... This orbital diagram for nitrogen shows how electrons fill the sub-orbitals. This is the electron configuration for the element sulfur, which has 16 electrons. We and everything around us are made ... The molecular orbital diagrams for molecules and ions are drawn from the order of increasing energies shown in the molecular orbital configuration. Always remember that the number of molecular orbitals formed must be equal to the number of atomic orbitals that were combined in the molecule.

1 Create The Atomic Orbital Of Diagram For Nitrogen 2 Construct The Orbital Diagram For Ni Homeworklib
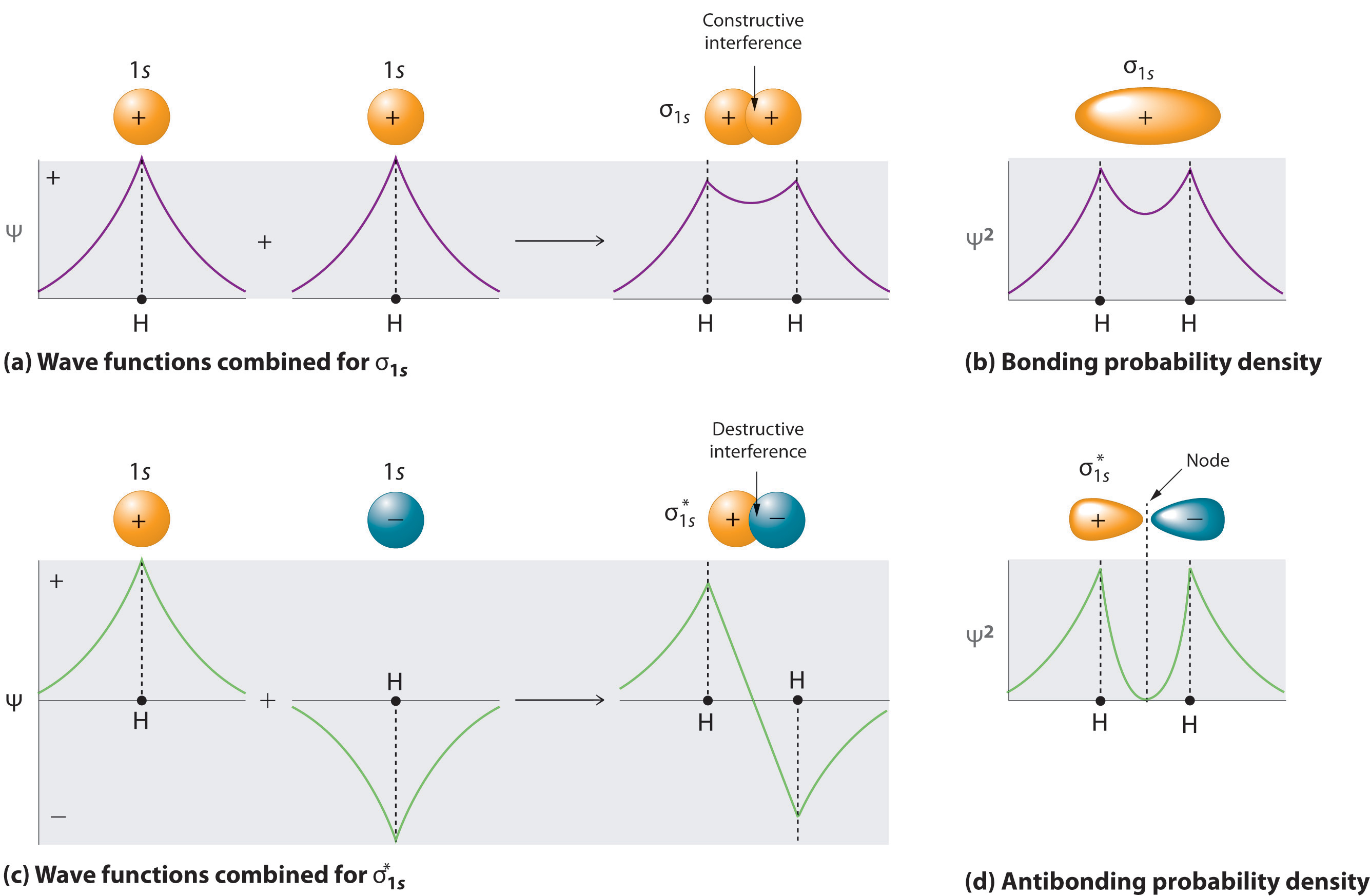
Molecular orbital diagram for nitrogen gas (N2)Use aufbau and Hund to fill with 10 valence electronsYou get sigma2s(2),sigma2s*(2),pi2p(4) ...
August 30, 2018 - Our videos prepare you to succeed in your college classes. Let us help you simplify your studying. If you are having trouble with Chemistry, Organic, Physics, Calculus, or Statistics, we got your back! Our videos will help you understand concepts, solve your homework, and do great on your exams.
The atomic number of Carbon is 6 so 2 electrons are filled in 's' orbital and the rest 4 are in the outer orbital that is why the valence number of electrons in carbon is 4. For Nitrogen, its atomic number is 7, so after 2 electrons occupy 's' orbital, the rest 5 are in the outer orbital so the valence number of electrons is 5.
April 5, 2018 - Our videos prepare you to succeed in your college classes. Let us help you simplify your studying. If you are having trouble with Chemistry, Organic, Physics, Calculus, or Statistics, we got your back! Our videos will help you understand concepts, solve your homework, and do great on your exams.
When we write the electron configuration of N the first two electrons go in the 1s orbital. As 1s can only hold 2 electrons and the other next ...
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as well as with other compounds.Oxygen is Earth's most abundant element, and after hydrogen and helium, it is the third-most abundant element in the universe.
Atomic no. Orbital Diagram of All Elements Diagrams; 1: Orbital diagram of Hydrogen (H) 2: Orbital diagram of Helium (He) 3: Orbital diagram of Lithium (Li) 4: Orbital diagram of Beryllium (Be) 5: Orbital diagram of Boron (B) 6: Orbital diagram of Carbon (C) 7: Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram ...
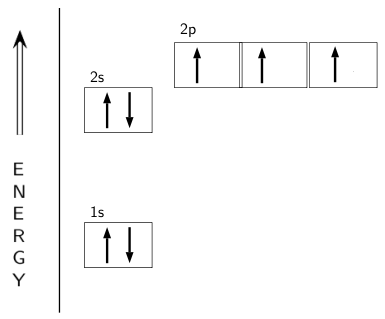
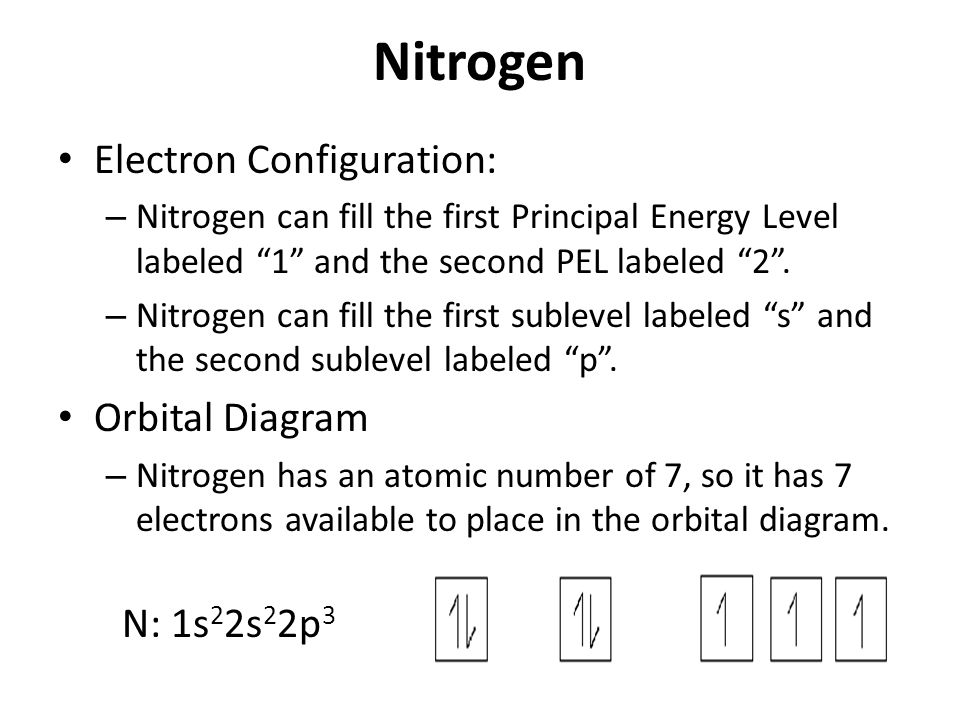
The electron configuration and orbital diagram for carbon are: Nitrogen (atomic number 7) fills the 1s and 2s subshells and has one electron in each of the three 2p orbitals, in accordance with Hund’s rule. These three electrons have unpaired spins. Oxygen (atomic number 8) has a pair of ...
The atomic number of nitrogen is 7 and its orbital electronic configuration is 1s 2 2s 2 2p 1 x 2p 1 y 2p 1 z. This shows that the nitrogen atom has three half-filled atomic orbitals. Two such atoms combine as a result of the overlap of the three half-filled orbitals and a triple bond gets formed (N = N). Formation of N 2 molecule. 6. Formation ...
Since 1s can only hold two electrons the next 2 electrons for N goes in the 2s orbital. Write out the orbital box diagram and the condensed electron configuration for Nitrogen. The atomic number of Nitrogen is 7 electronic configuration is 1s 2 2s 2 2p 3. Aufbau diagrams and spectroscopic electron configuration.
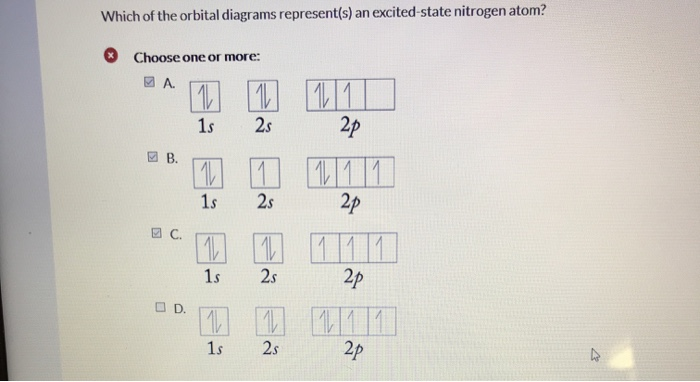
The choice A accurately specifies and illustrates the orbital diagram of a Nitrogen atom with 7 electrons. Based on the number of electrons in a Nitrogen atom, there are two energy levels, the s and p sub-levels: Nitrogen = 2, 5 . The first energy level, S will take up two electrons with opposite spin.
Two p-atomic orbitals (one from each nitrogen) atom combine to form two molecular orbitals, the bonding molecular orbital σ2px and antibonding molecular orbital σ*2px. The other four p-atomic orbitals (two from each nitrogen) atom combine to give four molecular orbitals, two bonding molecular orbitals i.e. π2py and π2pz, while two ...
As its atomic number is 7 it has 7 protons and for neutral nitrogen the number of protons is always equal to the number of electrons ie. In the ground state they are arranged in the electron configuration 1s 2 2s 2 2p 1 x 2p 1 y 2p 1 zIt therefore has five valence electrons in the 2s and 2p orbitals three of which the p-electrons are unpaired.
Carbon Electron Configuration: If you guys have come across our recent article then it would be easy for you all to understand the concept.But if you are new here and looking for the information related to the carbon element and its electronic configuration, then today we will help you with some of the things and if you will be here till the last line surely you will go with some knowledge ...
December 28, 2016 - In addition to listing the principle quantum number n and the subshell ell the orbital diagram shows all the different orientations and the...
In NH 2 molecule, the nitrogen atom is sp 3 - hybridized and one hybrid orbital possesses two electrons. Now to form an NH 3 molecule, three 1s- orbitals of three hydrogen atoms overlap with three sp 3 orbitals. The angle between H-N-H has to be 109.50 but as there is one occupied sp 3 - hybrid orbital, the angle
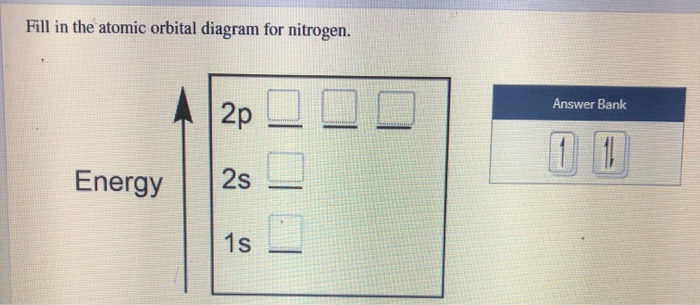
Nitrogen has atomic number 7. It is the seventh element of the periodic table having total 7 electrons. Two electrons will occupy the lowest energy subshell 1s then remaining 2 electrons will occupy 2s subshell and 3 electrons in 2p subshell. so the electronic configuration of nitrogen (N) is 1s 2 2s 2 2p 3 .
Orbital diagrams are pictorial descriptions of the electrons in an atom. Three guidelines are helpful in forming orbital diagrams. In response to the Auf Bau Precept, every electron occupies the bottom vitality orbital. You leap up a bit bit in vitality and we get the 2s orbital that make it the 2p sublevel.
Free essays, homework help, flashcards, research papers, book reports, term papers, history, science, politics
Learn about atomic orbital, the four quantum numbers (principal, angular momentum, magnetic, and spin), and how to write quantum numbers based on electron configuration. Updated: 08/18/2021 Create ...
The famous nitrogen molecule, N 2 , can also be perfectly described using molecular orbital diagrams: OM diagram for the N2 molecule. Source: Gabriel Bolívar. Note that this diagram is exactly the same as for the C 2 2- anion . This means that N 2 and C 2 2- are isoelectronic. However, this fact does not imply that both species behave in the ...
Electron Configuration For Nitrogen Ion. The atomic number of nitrogen is 7, the element nitrogen was discovered by a Scottish physician, Danial Rutherford. The year the element was discovered in the year 1772, through our article you will come to know about certain new things about the element Nitrogen.
Create the atomic orbital diagram for nitrogen. Start by adding the appropriate subshells. For example, boron is in the 2p block of the periodic table, and so you need to show the 2p subshell and everything below it. Next, click the orbitals to add...
1 week ago - The purpose of introducing quantum numbers has been to show that similarities in the electron arrangement or electron configuration lead to the similarities and differences in the properties of …
Compass · Tables · Index · Introduction · Professor Patricia Shapley, University of Illinois, 2012
We are told to create the atomic orbital diagram of Nitrogen. For this we will write the electronic configuration of Nitrogen atom which has 7...
Create the atomic orbital diagram for nitrogen. Source: upload.wikimedia.org 2 aufbau principle electrons are added one at a time to the lowest energy orbitals available until all the electrons in an atom have been accounted for fully.
In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons ...
Each He-atom contributes 2 electrons. Two electrons go into bonding molecular orbital σ (1s) and the remaining 2 go to antibondingσ* (1s) molecular orbital. The bond order for He 2 is absolutely zero i.e. 2 − 2/2= 0 and thus He 2 molecule is not formed. (ii) Nitrogen, N 2. Electronic configuration of N 2 molecule is
MODEL 2: ENERGY LEVEL DIAGRAM FOR THE NITROGEN ATOM 20 1 1 2s 11 Relative Energy 18.1 The electron configuration for the nitrogen atom is 1s22s22p?. The up and down arrows represent two different spin angular momentum states of the electron 6. Are two electrons with the same spin angular momentum described by the same atomic orbital in nitrogen?
Atomic Orbital Diagrams: These are also known as electron-in-a-box diagrams. This is a simplified diagram of how the electrons are arranged within the orbitals ...
March 25, 2020 - In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital. E none of the above. Orbital filling diagrams an orbital filling diagram is the more visual way to represent the arrangement of all the electrons in a particular atom.
Atomic number, atomic mass, protons, neutrons, electrons. Dřañ a bohr model of an oxygen atom in the space below. Label them with their charge. Determine the number of electrons in each of the atomic diagrams in model 1. "provide an orbital energy level diagram for the ground state of a nitrogen atom." in .
NF3 Lewis Structure, Molecular Geometry, Hybridization, Polarity, and MO Diagram. Nitrogen trifluoride or NF3 is a nitrogen halide compound that is slightly water-soluble. Its noticeable characteristics include being colorless and carrying a musty or moldy odor. NF3 has a molar mass of around 71.002 g/mol and a density of 3.003 kg/m3.
Nitrogen atomic number 7 fills the 1 s and 2 s subshells and has one electron in each of the three 2 p orbitals in accordance with Hunds rule. The number of isotopes possible b. Step 1 Find the symbol for the element on a periodic table.



















0 Response to "38 create the atomic orbital diagram for nitrogen."
Post a Comment