36 outer electron box diagram
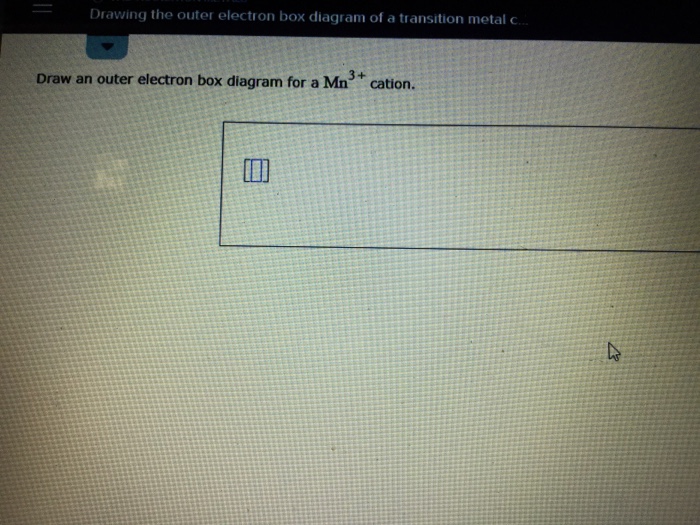
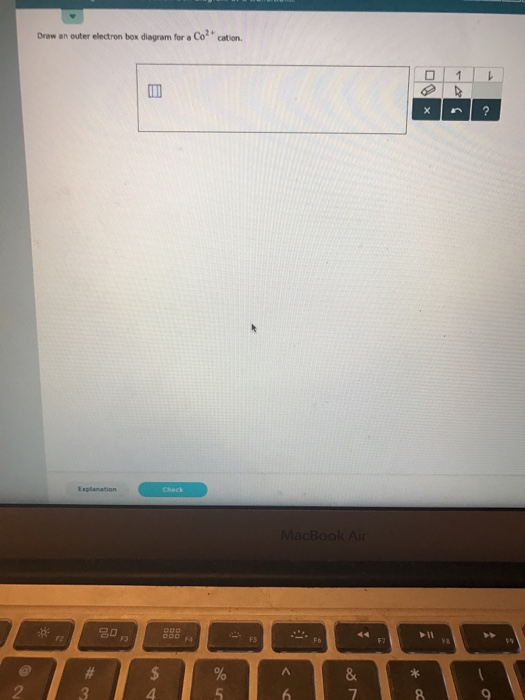
Draw an outer electron box diagram for a {eq}Mo^{2+}{/eq} cation. Transition Elements In the periodic table, elements are classified on the basis of their electronic configuration meaning the ... After the 4s is full we put the remaining four electrons in the 3d orbital and end with 3d4. Therefore the expected electron configuration for Chromium will be 1s 2 2s 2 2p 6 3s 2 3p 4 4s 2 3d 9. Note that when writing the electron configuration for an atom like Cr, the 3d is usually written before the 4s.
Electron Box diagrams of the outer electron arrangement and examples of the simple electron notation (e.g. 2.8.1) are also included, with brief comments in the end right hand column e.g. element symbol, group, series etc. The electrons-in-boxes notation for subshells: Boxes are used to represent an individual orbital or set of orbitals in ...

Outer electron box diagram
This sort of diagram is for representing the quantum numbers of the electrons in the system. The 3d boxes mean the quantum numbers n=3, l=2 with ml =-2,-1,0,+1,+2 for the 5 boxes. If a box has an up and a down arrow in it, this means there are 2 electrons with those quantum numbers, one with s=1/2, and one with 2=-1/2. Answer to: Draw an outer electron box diagram for a Nb^{3+} cation. By signing up, you'll get thousands of step-by-step solutions to your... Electron Configuration Exceptions. The periodic table can be a powerful tool in predicting the electron configuration of an element. However, we do find exceptions to the order of filling of orbitals shown in Figure 3 and Figure 4.For instance, the electron configurations (shown in Figure 6) of the transition metals chromium (Cr; atomic number 24) and copper (Cu; atomic number 29), among ...
Outer electron box diagram. About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators ... The electron configuration of Zn2+ is 1s22s22p63s23p63d10. Zinc is a d-block element, also known as a transition element. For the d-block elements, the outermost s-sublevel has higher energy than the d-sublevel, which is contrary to what the Aufbau diagram indicates. When d-block elements lose electrons, they lose the highest energy s electrons ... The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2. The helium atom contains two protons and two electrons. The first electron has the same four quantum numbers as the hydrogen atom electron ( n = 1, l = 0, ml = 0, ms = +1 2 m s = + 1 2 ). Electron configuration for Ru3+. Electron configuration for Ru 3+. What scientific concept do you need to know in order to solve this problem? Our tutors have indicated that to solve this problem you will need to apply the The Electron Configuration: Ions concept. You can view video lessons to learn The Electron Configuration: Ions.
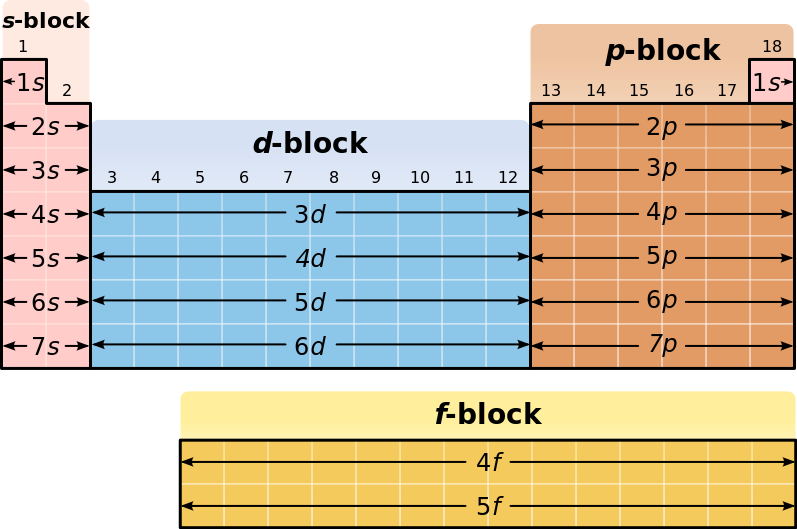
Your outer electrons are your electrons in the highest energy level so you would look at the largest coefficient in your electron configuration. Lastly your valence electrons is the last part of your condensed electron configuration. Ex: Electron Configuration for Manganese would be: 1s^2 2s^2 2p^6 3s^2 3p^6 3d^5 4s^2. Electron spin box diagrams of the outer electron orbitals for the electron configuration of the atom representing the superscripted electrons beyond the . The electrons fill into the orbitals following the order 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p, with each s orbital holding . The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2. The helium atom contains two protons and two electrons. The first electron has the same four quantum numbers as the hydrogen atom electron ( n = 1, l = 0, ml = 0, ms = +1 2 m s = + 1 2 ). Correct Electron Configuration for Copper (Cu) Half-filled and fully filled subshell have got extra stability. Therefore, one of the 4s2 electrons jumps to the 3d9. This give us the (correct) configuration of: 1s2 2s2 2p6 3s2 3p6 3d10 4s1. For the Cu+ ion we remove one electron from 4s1 leaving us with: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10
This notation uses a box to represent the orbital, the label for the orbital and an arrow to represent the electron. The electronic configuration for hydrogen can be written as 1s 1.This is a short-hand notation which identifies the level, the sublevel and the number of electrons in the sublevel. Box spin diagram of outer electron orbitals for the electron configuration of the 15 Phosphorus, P, 1s22s22p63s23p3 (), [Ne]3s 3p, P, p-block, Gp5/ Build the orbital diagram for the ion most likely formed by phosphorus? a \ will be an arrow going one way and a / will be the other way. a [ ] represents a box. Electron Configuration Calculator Added Nov 11, 2014 by Brennenlb in Chemistry Find the Electron configuration of any Element on the Periodic Table of Elements with this simple, yet very useful widget. Valence Electrons & Bohr Diagrams Atomic Structure Atoms have a nucleus that contains Protons and Neutrons Electrons are contained in shells that surround the nucleus An atom is made of mostly empty space Protons have a positive charge Electrons have a negative charge Neutrons are Neutral Valence Electrons Each electron shell can hold a certain number of electrons Electron shells are filled ...
Refer to the explanation. The electron configuration of manganese, atomic number 25, is "1s"^2"2"^2"2p"^6"3s"^2"3p"^6"3d"^5"4s"^2". The diagram below represents the electron configuration as an orbital diagram.
About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators ...
Box spin diagram of outer electron orbitals for the electron configuration of the 15 Phosphorus P 1s22s22p63s23p3 Ne3s 3p P pblock Gp5 Build the orbital diagram for the ion most likely formed by phosphorus. The rules for orbital filling diagrams. Energy 0 1 1 x 5. Now this is only one way we can draw the electron dot diagram for Oxygen.
The eight valence electrons, a full outer s and p sublevel, give the noble gases their special stability. When examining chemical bonding, it is necessary to keep track of the valence electrons of each atom. Electron dot diagrams are diagrams in which the valence electrons of an atom are shown as dots distributed around the element's symbol ...
Krypton Orbital Diagram. Diagram of the nuclear composition, electron configuration, chemical data, and valence orbitals of an atom of krypton (atomic number: 36), the most common . Box spin diagram of outer electron orbitals for the electron configuration of the atom . 36 Krypton, Kr, [Ar]3ds24p6 = [Kr] (), [Ar]3d 4s 4p v. stable, Kr .
4+ Draw an outer electron box diagram for a Cr* cation. Question: 4+ Draw an outer electron box diagram for a Cr* cation. This problem has been solved! See the answer See the answer See the answer done loading. Show transcribed image text Expert Answer. Who are the experts?
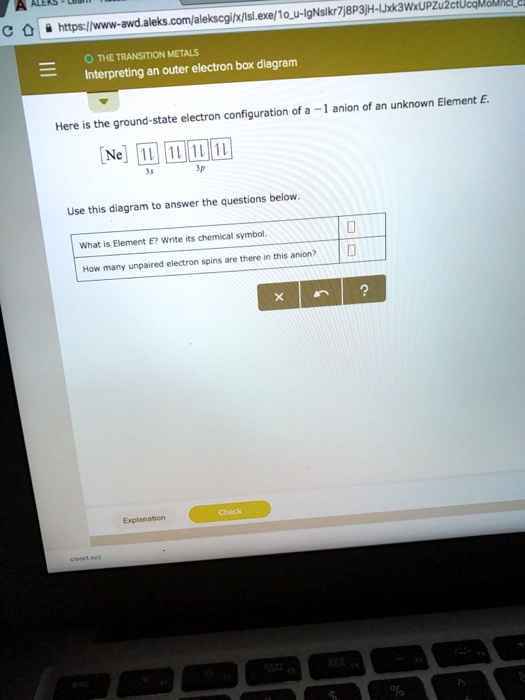
Solved Comfalekscgxislexe 1o U Lgnslkr7j8p3 H4 Lxkiwxupzuzctuca Https Ilwww Awd Aleks The Transition Metals Interpreting An Outer Electron Box Diagram Anion An Unknown Element Ground State Electron Configuration Of Here Ne Answer The Questions
Draw an outer electron box diagram for a CO^2+ cation. Question: Draw an outer electron box diagram for a CO^2+ cation. This problem has been solved! See the answer See the answer See the answer done loading. Show transcribed image text Expert Answer. Who are the experts?
Neon electron configuration is 1s 2 2s 2 2p 6.The symbol for neon is 'Ne' and it is an inert element. This article gives an idea about the electron configuration of neon(Ne) and orbital diagram, period and groups, valency and valence electrons of neon, application of different principles. The tenth element in the periodic table is neon.

Solved Part C Identify The General Outer Electron Configuration For Each Group Of Elements Shown In Brainly Com
Fe2+ Orbital Diagram. For midterm question Q5C, why the electron configuration for Fe2+ is not [Ar]3d^5 4S^1? The outermost shell, in this case, is the 4s orbital. You're removing 2 electrons from it to generate the Fe2+ ion, which are removed from the 4s orbital first (this is always the case in transition chemistry - as far as.

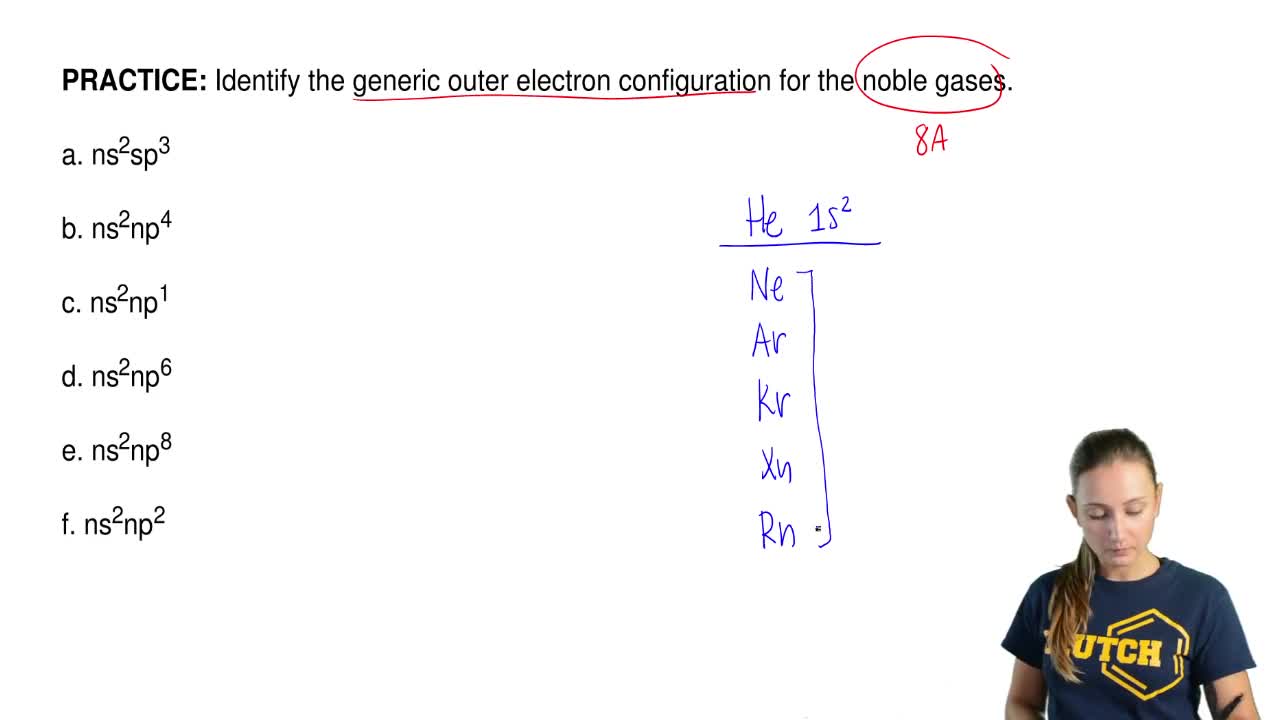
Identify The Generic Outer Electron Configuration For The Alkaline Earth Metals Ns2np3 Ns2np4 Ns2 Ns2np1 Ns1 Homeworklib
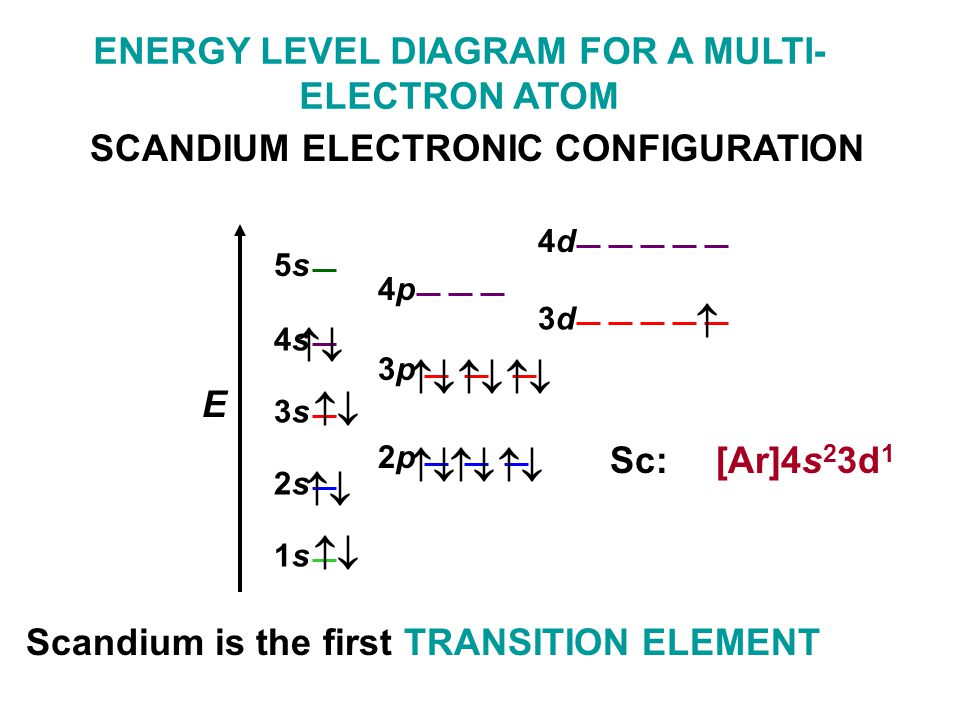
159 ENERGY DIAGRAM - We can map out electrons around an atom using an energy diagram: E N E R G Y 1s 2s 2p 3s 3p 3d 4s 4p 4d 5s 5p ... so that it has a share in eight electrons in its outer shell! 163 ELECTRON CONFIGURATION - A shorthand way to write about electron arrangement around an atom. Shell and
Orbital diagrams make use of a box, circle, or line for each ... same outer electron configuration. ... Chlorine reacting with potassium. 8-24. Table 8.3 Partial Orbital Diagrams and Electron Configurations * for the Elements in Period 4. * Colored type indicates the sublevel to which the last electron is added.

Identify The Group Number And Generic Outer Electron Configuration For A Neutral Main Group Element Brainly Com
["Kr"]4d^10 Your starting point here will be the electron configuration of a neutral cadmium atom. Cadmium, "Cd", is located in period 5, group 12 of the periodic table and has an atomic number equal to 48. This means that a neutral cadmium atom will have a total of 48 electrons surrounding its nucleus. This also tells you that the "Cd"^(2+) cation, which has two electrons less than the ...
Electron Configuration Exceptions. The periodic table can be a powerful tool in predicting the electron configuration of an element. However, we do find exceptions to the order of filling of orbitals shown in Figure 3 and Figure 4.For instance, the electron configurations (shown in Figure 6) of the transition metals chromium (Cr; atomic number 24) and copper (Cu; atomic number 29), among ...
Answer to: Draw an outer electron box diagram for a Nb^{3+} cation. By signing up, you'll get thousands of step-by-step solutions to your...
This sort of diagram is for representing the quantum numbers of the electrons in the system. The 3d boxes mean the quantum numbers n=3, l=2 with ml =-2,-1,0,+1,+2 for the 5 boxes. If a box has an up and a down arrow in it, this means there are 2 electrons with those quantum numbers, one with s=1/2, and one with 2=-1/2.
The Outer Electronic Configuration Of Gd Atomic No 64 Is Sarthaks Econnect Largest Online Education Community

Electron Configurations How To Write Out The S P D F Electronic Arrangements Of Atoms Ions Periodic Table Oxidation States Using Orbital Notation Gce A Level Revision Notes










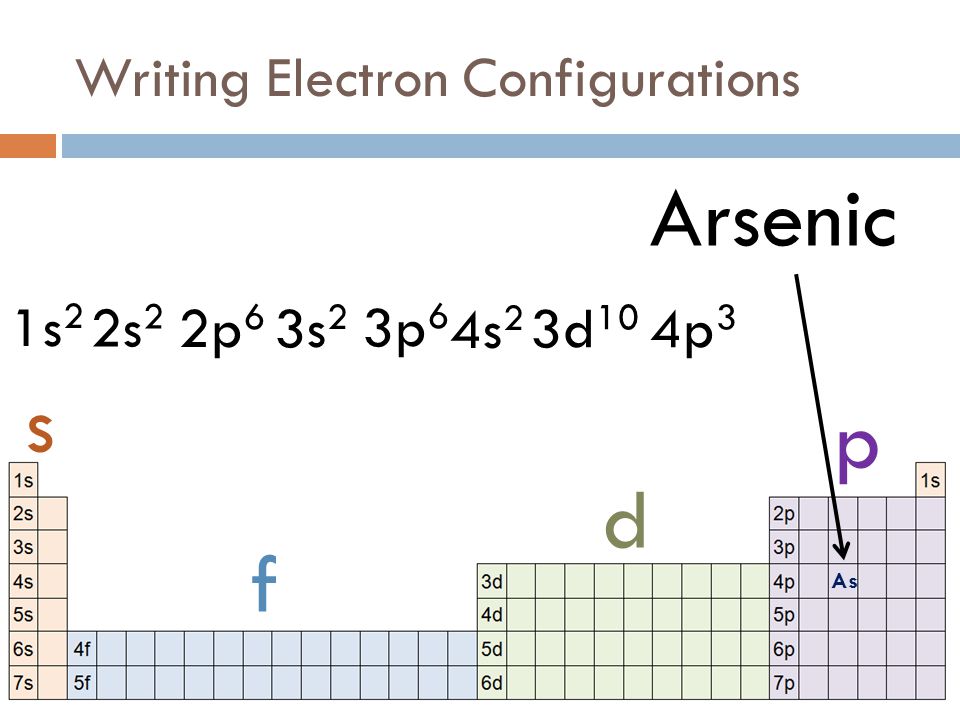




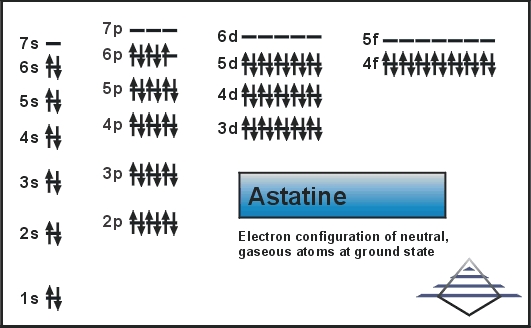

0 Response to "36 outer electron box diagram"
Post a Comment