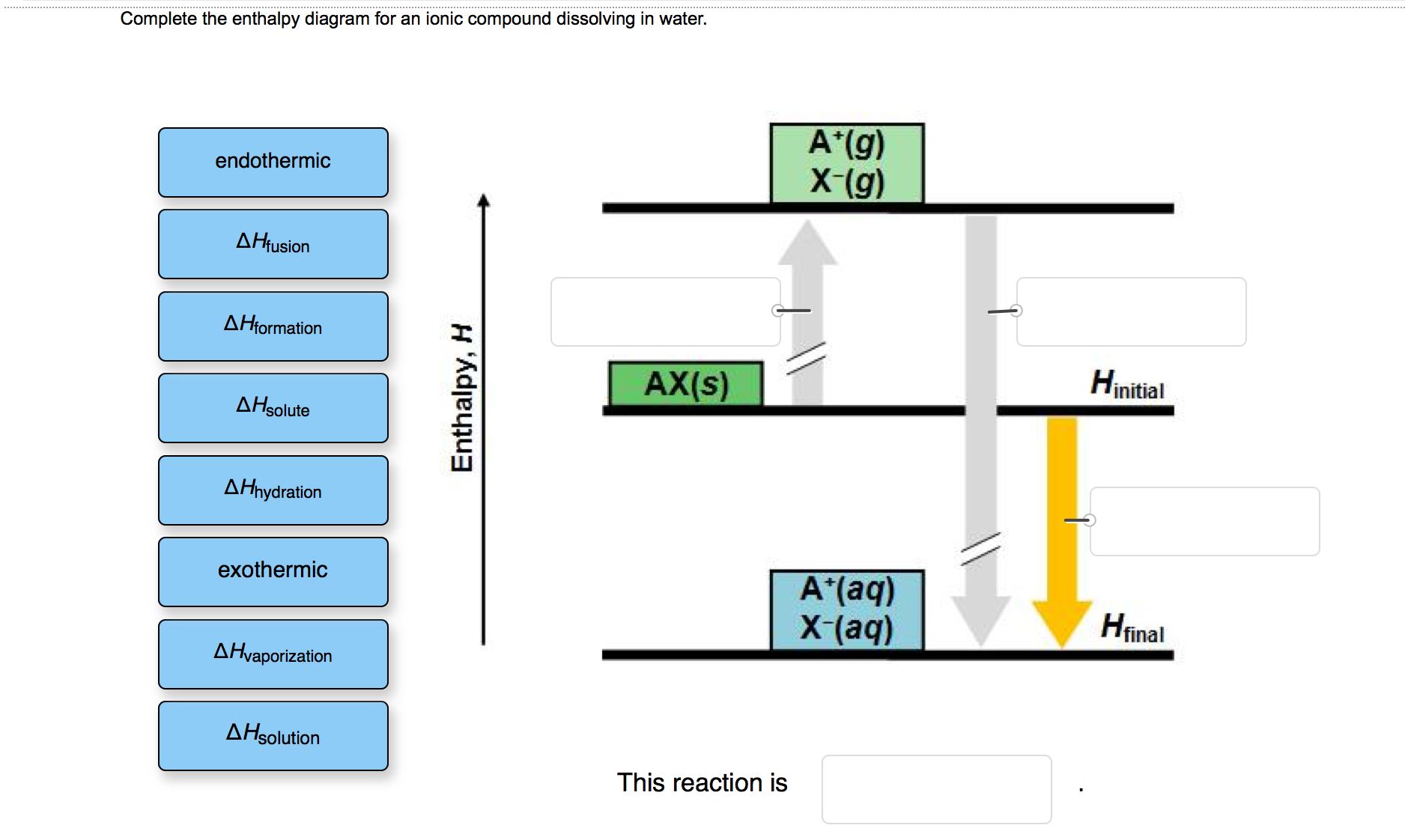
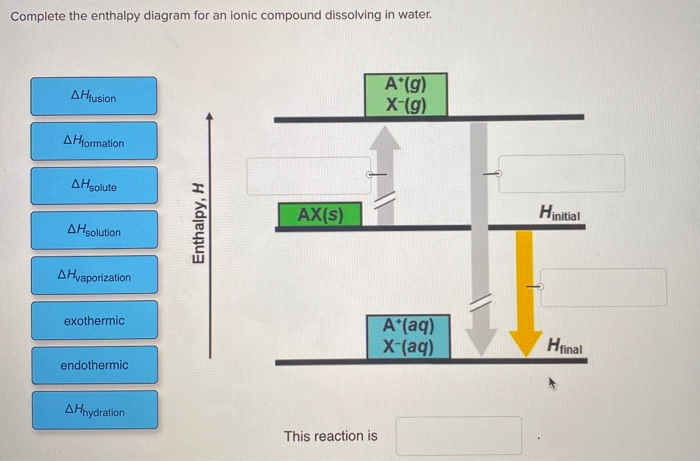
40 complete the enthalpy diagram for an ionic compound dissolving in water.
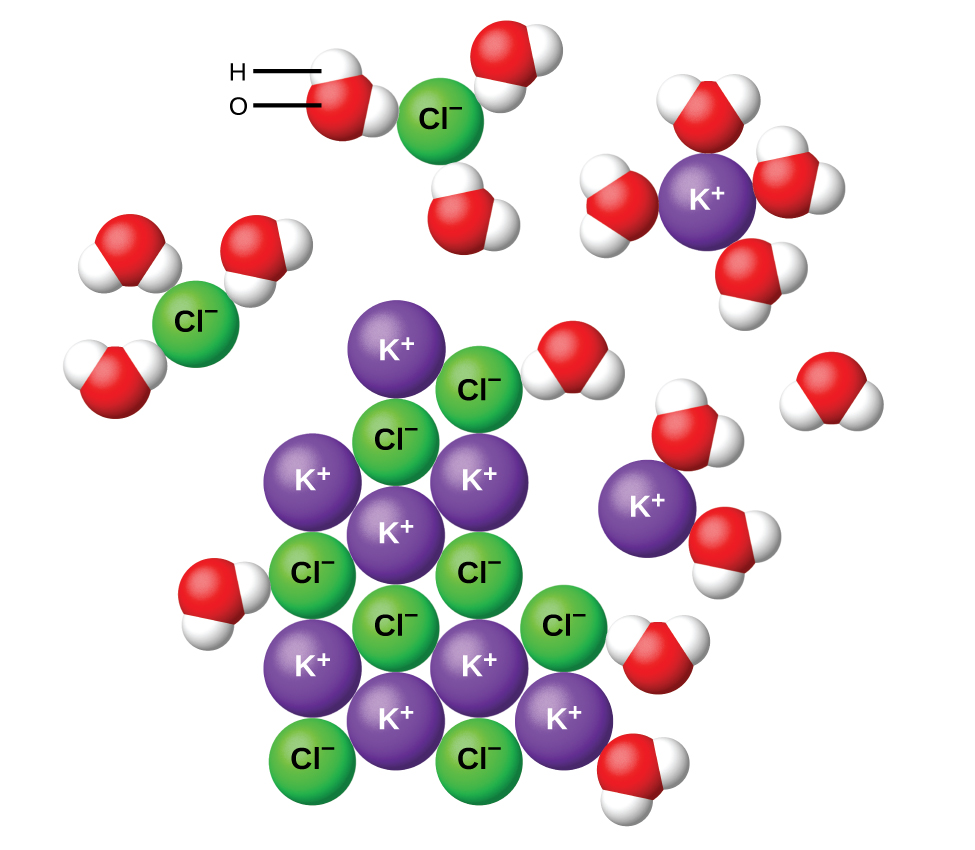
greetsieler-grachten-stern.de › sodium-carbonate-andSodium carbonate and hydrochloric acid reaction type Mar 19, 2022 · Barbie pegasusun sihri tek parça türkçe dublaj izle Not defteri lisans Eylül barbie pegasusun sihri izle quizlet.com › 481131056 › chem-1308-dr-m-jiangChem 1308 - Dr. M Jiang (Spring 2020) Ch 11 - 13 - Quizlet .When an ionic compound dissociates, the ions are separated from each other and surrounded by water molecules. In this compound, the sodium ion separates from the chloride ion:NaCl(aq)→Na+(aq)+Cl−(aq)NaCl(aq)→NaX+(aq)+ClX−(aq)Instead of assuming complete dissociation, this problem presents the van't Hoff factor, which tells us that the ...
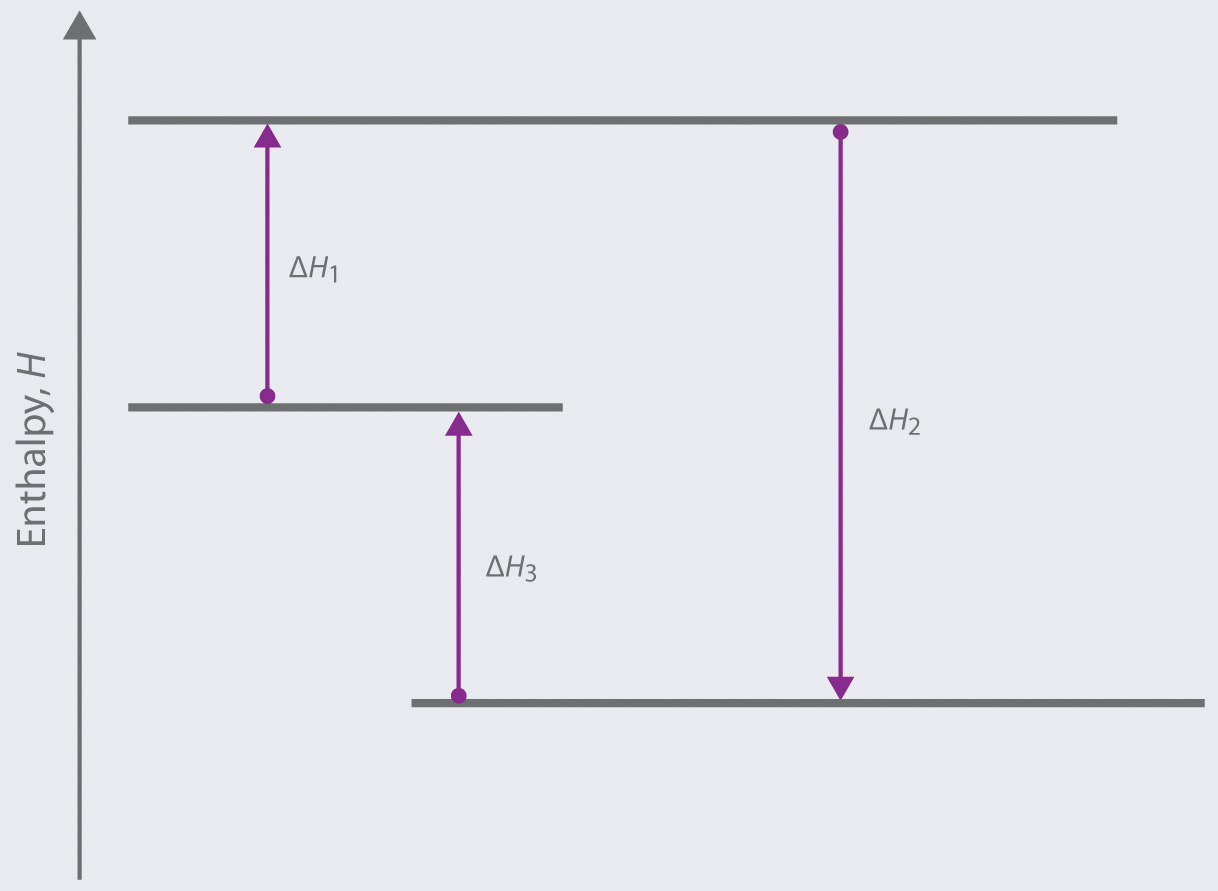
Solved Complete the enthalpy diagram for an ionic compound ... Chemistry. Chemistry questions and answers. Complete the enthalpy diagram for an ionic compound dissolving in water B (g) Y (g) AHsolution B' (aq) Y- (aq) Ahydration Ht final exothermic H solute AHvaporization BY (S) Hinitial endothermic Hormation This reaction is.
Complete the enthalpy diagram for an ionic compound dissolving in water.
complete the enthalpy diagram for an ionic compound ... complete the enthalpy diagram for an ionic compound dissolving in water. Answer. + 20. Watch. 1. Solubility Rules of Ionic Solids in Water - ThoughtCo This is a list of the solubility rules for ionic solids in water. Solubility is a result of an interaction between polar water molecules and the ions that make up a crystal. Two forces determine the extent to which the solution will occur: Solubility - Purdue University We can generally assume that salts dissociate into their ions when they dissolve in water. Ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic bonds in the solid and the energy required to separate the water molecules so that the ions can be inserted into solution.
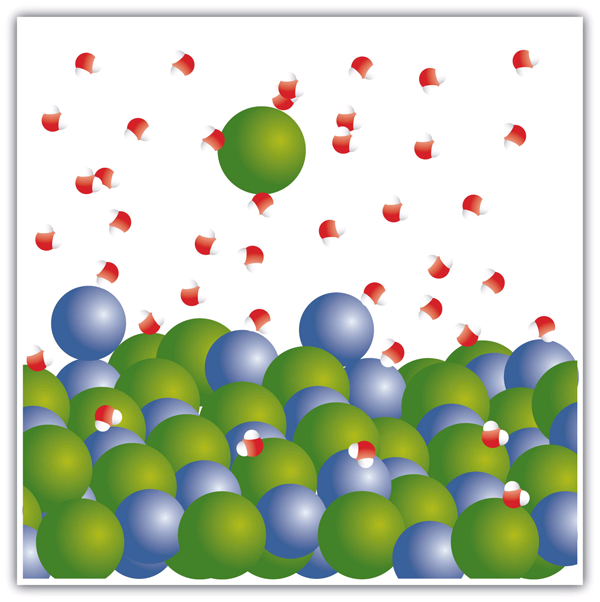
Complete the enthalpy diagram for an ionic compound dissolving in water.. haptotherapie-west.nl › virtual-chemistry-lab-answershaptotherapie-west.nl Our connect chem answers are 100% correct and affordable too! Our tutors are available 24*7 Virtual Water Testing Lab #21. One of the first concepts that I teach in Forensics is the microscope . A List of Basic Chemistry Apparatus. During Session 3, one of the physical changes we examined was dissolving, particularly dissolving salt in water. How does sodium chloride (NaCl) dissolve in water? Sodium chloride (NaCl) dissolves when water molecules continuously attack the NaCl crystal, pulling away the individual sodium (Na +) and chloride (Cl -) ions. This nonstop attack continuous until the whole NaCl crystal disintegrates. To understand this process at the molecular level, we must apply the three steps we previously discussed. Dissolving Process | Chemistry for Non-Majors The Dissolving Process. Water typically dissolves many ionic compounds and polar molecules. Nonpolar molecules such as those found in grease or oil do not dissolve in water. We will first examine the process that occurs when an ionic compound such as table salt (sodium chloride) dissolves in water. Water molecules move about continuously due to ... Dissociation of Sodium Chloride in Water | Reactions in ... Dissociation of Sodium Chloride in Water. It is the polar nature of water that allows ionic compounds to dissolve in it. In the case of sodium chloride (\(\text{NaCl}\)) for example, the positive sodium ions (\(\text{Na}^{+}\)) are attracted to the negative pole of the water molecule, while the negative chloride ions (\(\text{Cl}^{-}\)) are attracted to the positive pole of the water molecule.
Solved Which box on the left fits into which space on the ... Transcribed image text: Complete the enthalpy diagram for an ionic compound dissolving in water. endothermic delta H fusion delta H formation delta H solute delta H hydration exothermic delta H vaporization delta H solution This reaction is opentextbc.ca › chemistry › chapter4.5 Quantitative Chemical Analysis – Chemistry In a combustion analysis, a weighed sample of the compound is heated to a high temperature under a stream of oxygen gas, resulting in its complete combustion to yield gaseous products of known identities. The complete combustion of hydrocarbons, for example, will yield carbon dioxide and water as the only products. › indexCells and cell structure quiz questions - Footprints-Science ... The lungs Quiz States of matter Quiz Chromatography Quiz GCSE Biology sample animations and quizzes GCSE Chemistry sample animations and quizzes GCSE Physics sample animations and quizzes GCSE Investigative Skills animations/slides Sodium carbonate and hydrochloric acid reaction type 2022-03-19 · Barbie pegasusun sihri tek parça türkçe dublaj izle Not defteri lisans Eylül barbie pegasusun sihri izle
The Dissolving of Solid Sodium Hydroxide in Water ... NaOH (s) + H2O (l) -> Na+ (aq) + OH- (aq) 2. Calculate the number of moles of sodium hydroxide dissolved. Show your work. 3. Calculate the amount of energy involved in this dissolving process. Show your work. qsurroundings = -qsystem qsystem = -3. 126kJ 4. Determine the enthalpy change, per mole of sodium hydroxide dissolved. 4.5 Quantitative Chemical Analysis – Chemistry The complete combustion of hydrocarbons, for example, will yield carbon dioxide and water as the only products. The gaseous combustion products are swept through separate, preweighed collection devices containing compounds that selectively absorb each product . The mass increase of each device corresponds to the mass of the absorbed product and may be used in … Solved Complete the enthalpy diagram for an ionic compound ... Complete the enthalpy diagram for an ionic compound dissolving in water. A (g) X-(g) AHnydration AHusion exothermic AX(s) initial ??,aporization endothermic A (aq) x-(aq) A Hsolution Hrinal ??,ormation AHsolute This reaction is ; Question: Complete the enthalpy diagram for an ionic compound dissolving in water. A (g) X-(g) AHnydration AHusion ... Chem 1308 - Dr. M Jiang (Spring 2020) Ch 11 - 13 - Quizlet .When an ionic compound dissociates, the ions are separated from each other and surrounded by water molecules. In this compound, the sodium ion separates from the chloride ion:NaCl(aq)→Na+(aq)+Cl−(aq)NaCl(aq)→NaX+(aq)+ClX−(aq)Instead of assuming complete dissociation, this problem presents the van't Hoff factor, which tells us that the ions only …
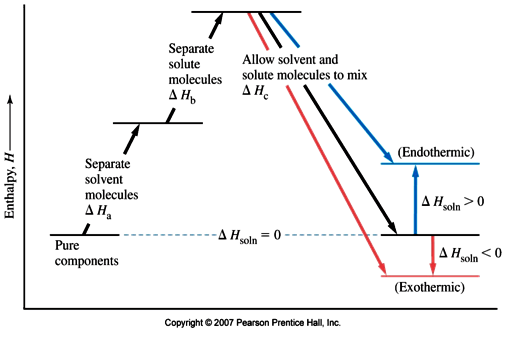
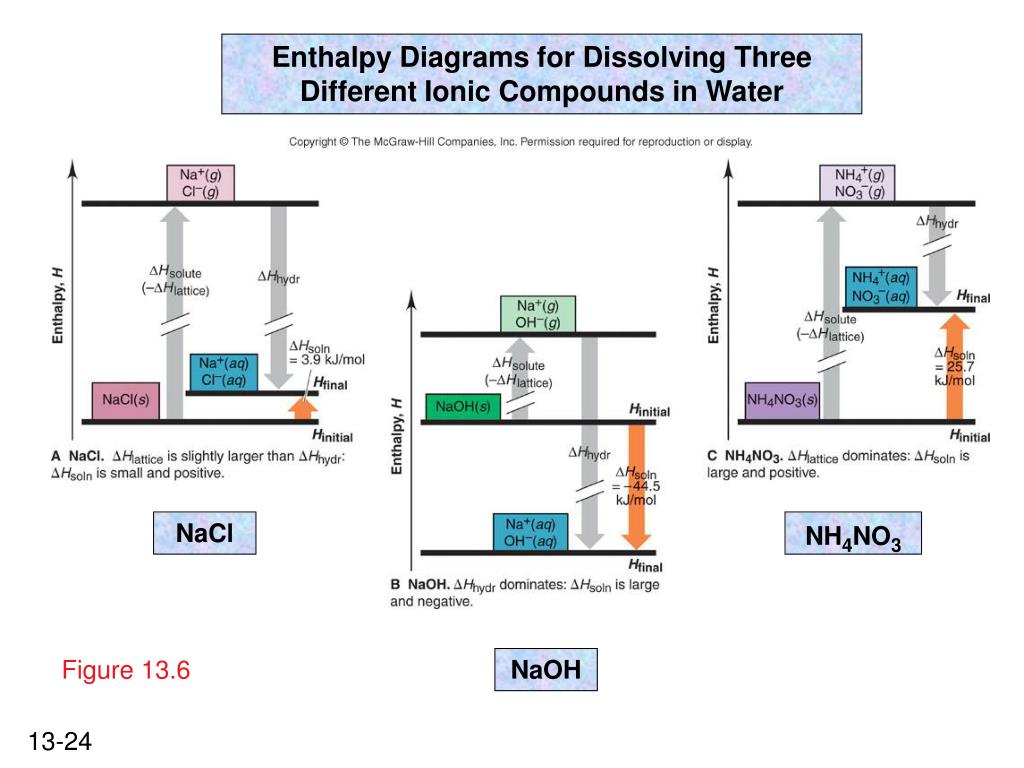
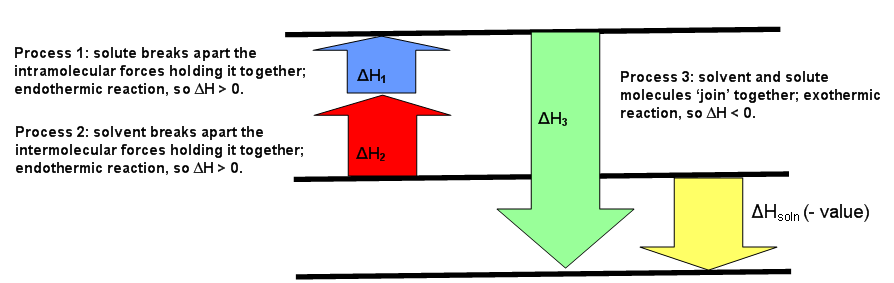
Complete the enthalpy diagram for an ionic compound ... The diagram represents the solution process (lattice energy, heat of hydration, and heat of solution) for an ionic compound dissolving in water. ht Arrow Brepresents enthalpy of mixing and is exothermic.
Why do ionic compounds dissolve in water? | Socratic Ionic compounds dissolve in water because the water molecules hydrate the ions. > To dissolve an ionic compound, the water molecules must be able to stabilize the ions that result from breaking the ionic bond. They do this by hydrating the ions. Water is a polar molecule. It has a permanent dipole. The "O" atom has a partial negative charge, and the "H" atoms have a partial positive charge.
Lab 11 - Thermodynamics of Salt Dissolution Some ionic compounds give off heat when dissolving in water and others absorb heat. Whether the dissolution process of a given ionic compound gives off or absorbs heat depends on the strength of the intermolecular forces holding the solid together, as well as those between the ions and the water once dissolved.
Water molecules and their interaction with salt | U.S ... Water molecules pull the sodium and chloride ions apart, breaking the ionic bond that held them together. After the salt compounds are pulled apart, the sodium and chloride atoms are surrounded by water molecules, as this diagram shows. Once this happens, the salt is dissolved, resulting in a homogeneous solution.
Solved Complete the enthalpy diagram for an ionic compound ... Chemistry. Chemistry questions and answers. Complete the enthalpy diagram for an ionic compound dissolving in water. exothermic B* (9) Y- (g) AHvaporization AHsolute B* (aq) Y- (aq) endothermic Enthalpy, H Hfinal Anydration Arusion BY (s) A Hormation Hinitial AH solution This reaction is.
Sodium hydroxide - Wikipedia Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula NaOH. It is a white solid ionic compound consisting of sodium cations Na + and hydroxide anions OH −. Sodium hydroxide is a highly caustic base and alkali that decomposes proteins at ordinary ambient temperatures and may cause severe chemical burns.It is highly …
11.2 Electrolytes - Chemistry When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. These substances constitute an important class of compounds called electrolytes.Substances that do not yield ions when dissolved are called nonelectrolytes.If the physical or chemical process that generates the ions is essentially 100% efficient (all of the dissolved ...
PDF Lattice Enthalpy NEW - WordPress.com Enthalpy change of solution and Enthalpy change of Hydration When ionic compounds dissolve in water, there is usually a temperature change. Sometimes this is exothermic (e.g. dissolving calcium chloride) and sometimes endothermic (e.g. dissolving ammonium nitrate). The experiments are easy to carry out in a laboratory, and the usual equations ...
Properties of Ionic and Covalent Compounds - A Plus Topper Hence, ionic compounds cannot conduct electricity in the solid state. The electrical conductivity of ionic compounds in the molten (liquid) and aqueous states can be explained below: When the ionic compounds are melted through heating or dissolved in water, the positive and negative ions will break free and become mobile, that is able to move ...
Temperature Changes in Dissolving | Chapter 5: The Water ... When water dissolves a substance, the water molecules attract and "bond" to the particles (molecules or ions) of the substance causing the particles to separate from each other. The "bond" that a water molecule makes is not a covalent or ionic bond. It is a strong attraction caused by water's polarity.
Comparison between Covalent and Ionic Compounds ... At room temperature and normal atmospheric pressure, covalent compounds may exist as a solid, a liquid, or a gas, whereas ionic compounds exist only as solids. Although solid ionic compounds do not conduct electricity because there are no free mobile ions or electrons, ionic compounds dissolved in water make an electrically conductive solution.
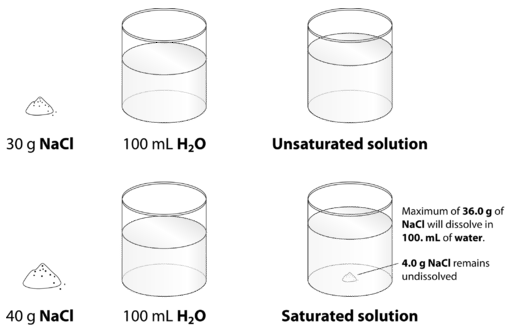
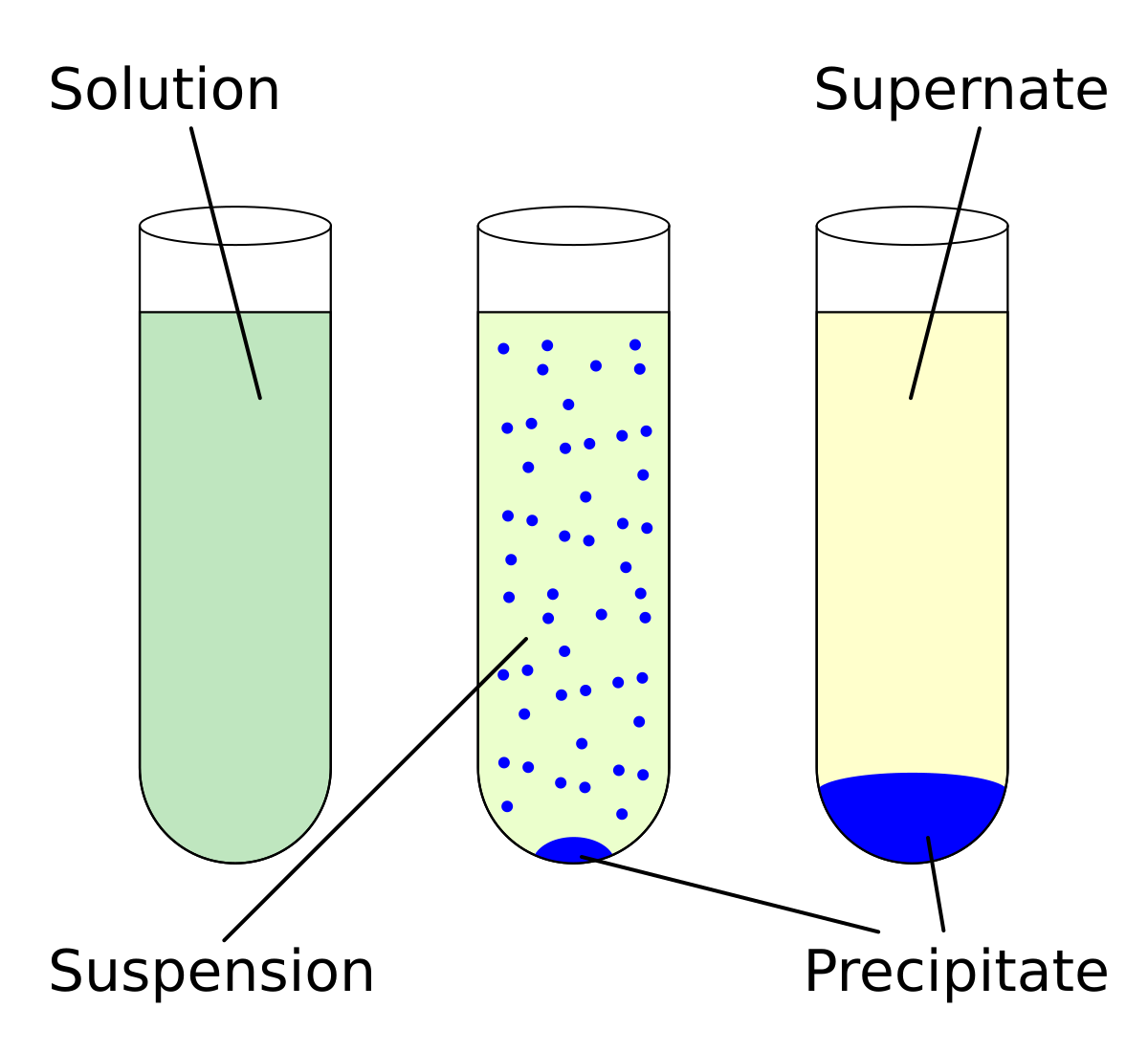
Solubility - Wikipedia In even more simple terms a simple ionic compound (with positive ... For example, a mixture of salt (sodium chloride) and silica may be separated by dissolving the salt in water, and filtering off the undissolved silica. The synthesis of chemical compounds, by the milligram in a laboratory, or by the ton in industry, both make use of the relative solubilities of the desired product, as well …
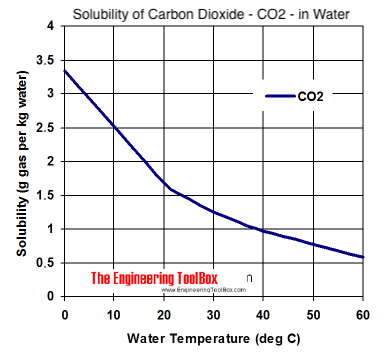
dink-magazin.de › the-heat-is-on-answer-keydink-magazin.de The heat loss due to evaporation of water from a surface of an open tank is dominant at higher water temperatures. (b) It is a good conductor of heat. It might be necessary to press the fn (function) key at the same time to activate the backlight key. 16 de set. When heat transfers through the heater or AC in your house.
Hot and Cold Packs: A Thermochemistry Activity - Carolina.com Many instant hot and cold packs function by dissolving a salt into water. As the salt disassociates, heat is either released in an exothermic reaction or absorbed in an endothermic reaction. Commercial instant cold packs typically use either ammonium nitrate or urea as their salt component; hot packs often use magnesium sulfate or calcium chloride. These reactions …
CHAPTER 2.0 THERMOCHEMISTRY_NOTES & TUTORIAL Q's - Flip ... When an ionic compound dissolves in water, positive end of water molecule will attract the negative ion & negative end of water will attract the positive ion DISSOLUTION OF IONIC SOLID INVOLVES 2 STEPS Step 1 Dissociation of lattice : Energy absorbed is the lattice dissociation energy MX(s) H2O M+ (g) + X− (g) Step 2
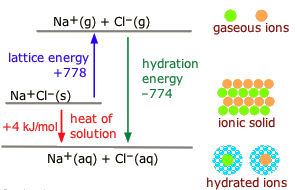
Ionicity diagram - Big Chemical Encyclopedia The energy diagram for the formation of this hypothetical compound follows the pattern of that for NaCl but an additional endothermic step is added for the second ionisation energy of sodium. The lattice energy is calculated on the assumption that the compound is ionic and that Na is comparable in size with Mg ".
The Cold Pack: A Chilly Example of an ... - Let's Talk Science 2020-06-01 · Chemical dissociation of solid ammonium nitrate in water to form aqueous ammonium and aqueous nitrate (©2020 Let’s Talk Science). Dissolving an ionic compound, like table salt or ammonium nitrate, involves energy. Like other types of reactions, heat energy may be given off or taken in when the material dissolves. This energy is called the
Study Guide and Solutions Manual to ... - Academia.edu Study Guide and Solutions Manual to Accompany T.W. Graham Solomons / Craig B. Fryhle / Scott A. Snyder / Jon Antilla
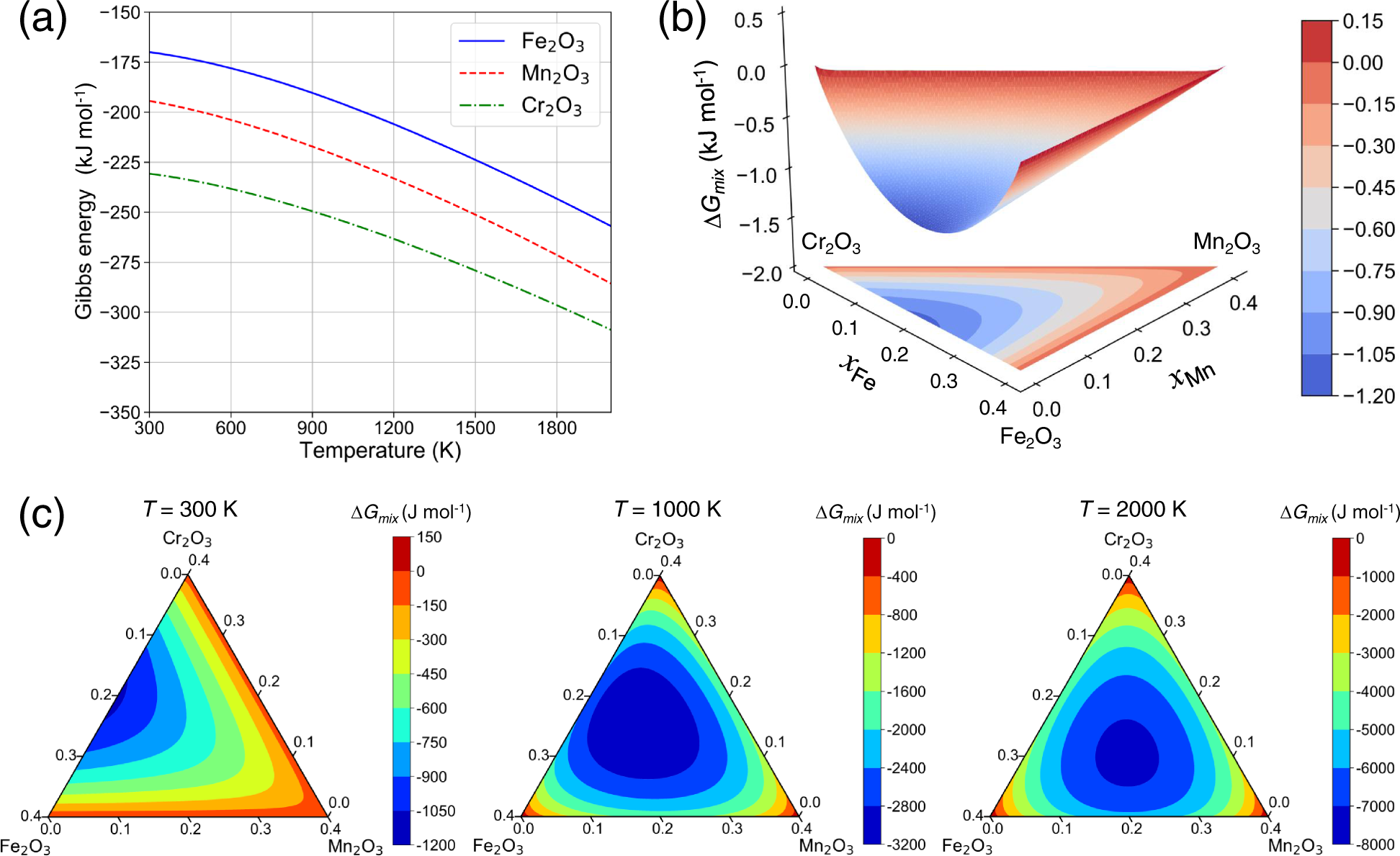
PDF (3) CE = 0 if O2− or water ionic or H bonding 1 (f) Magnesium oxide reacts with water / forms Mg(OH) 2 Allow MgO does not dissolve in water / sparingly soluble / insoluble 1 [11] Q3. (a) Enthalpy change for the formation of 1 mol of gaseous atoms allow heat energy change for enthalpy change 1 From the element (in its standard state)
Fractional distillation animation - Footprints-Science ... The lungs Quiz States of matter Quiz Chromatography Quiz GCSE Biology sample animations and quizzes GCSE Chemistry sample animations and quizzes GCSE Physics sample animations and quizzes GCSE Investigative Skills animations/slides
(Get Answer) - Compound XY is an ionic compound that ... Compound XY is an ionic compound that dissociates as it dissolves in water. The lattice energy of XY is -600 kJ mol-1.The hydration energy of its ions is -610 kJ mol-1. (a) Write the thermochemical equations for the two steps in the formation of a solution of XY in water.
State of Matter Questions and Answers - Study.com If you wanted to synthesize a compound that had a fairly low melting point and a low density, you would want to synthesize: a. a metallic solid b. a covalent solid c. an ionic solid d. a molecular ...
How to Draw & Label Enthalpy Diagrams - Video & Lesson ... An enthalpy diagram is a method used to keep track of the way energy moves during a reaction over a period of time. Learn how to draw and label enthalpy diagrams, the definition of an enthalpy ...
Ionic Bond - Definition, Types, Properties & Examples Carbon dioxide, water, chlorine gas are some common examples of compounds having a covalent bond. On the other hand, few compounds like table salt, magnesium oxide, and calcium chloride are ionic. But in reality, no bond or compound is completely ionic or covalent in nature.
Solved Complete the enthalpy diagram for an ionic compound ... Answer : The given is the complete enthalpy diagram for the ionic compound. The AX (s) is separated into its ion in gaseous forms that is A+ (g) and X- (g). This will require the solute. When the …. View the full answer. Transcribed image text: Complete the enthalpy diagram for an ionic compound dissolving in water.
Answered: How does water dissolve polar and ionic… | bartleby 9.1 Liquids, Solids, And Intermolecular Forces 9.2 Vaporization And Condensation 9.3 Vapor Pressure 9.4 Solids And Changes Of Phase 9.5 Water: Its Important And Unusual Properties 9.6 Crystalline Solids 9.7 Network Solids 9.8 Materials Science 9.9 Metals, Semiconductors, And Insulators 9.10 Silicon And The Chip 9.11 Cement, Ceramics, And Glass ...
Water | Facts, Properties, Structure, Compounds & Summary The only difference is that ionic compounds are broken down into individual ions while in the case of non-ionic polar substances, hydration shells are formed around the entire molecule keeping its covalent bonds intact. Non-Polar Compounds. Unfortunately, water proves to be a poor solvent for non-ionic, non-polar compounds due to its polarity.
Solubility - Purdue University We can generally assume that salts dissociate into their ions when they dissolve in water. Ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic bonds in the solid and the energy required to separate the water molecules so that the ions can be inserted into solution.
Solubility Rules of Ionic Solids in Water - ThoughtCo This is a list of the solubility rules for ionic solids in water. Solubility is a result of an interaction between polar water molecules and the ions that make up a crystal. Two forces determine the extent to which the solution will occur:
complete the enthalpy diagram for an ionic compound ... complete the enthalpy diagram for an ionic compound dissolving in water. Answer. + 20. Watch. 1.




:max_bytes(150000):strip_icc()/salt-shaker--close-up-sb10069325p-001-5a4d04ef845b340037b40fca.jpg)








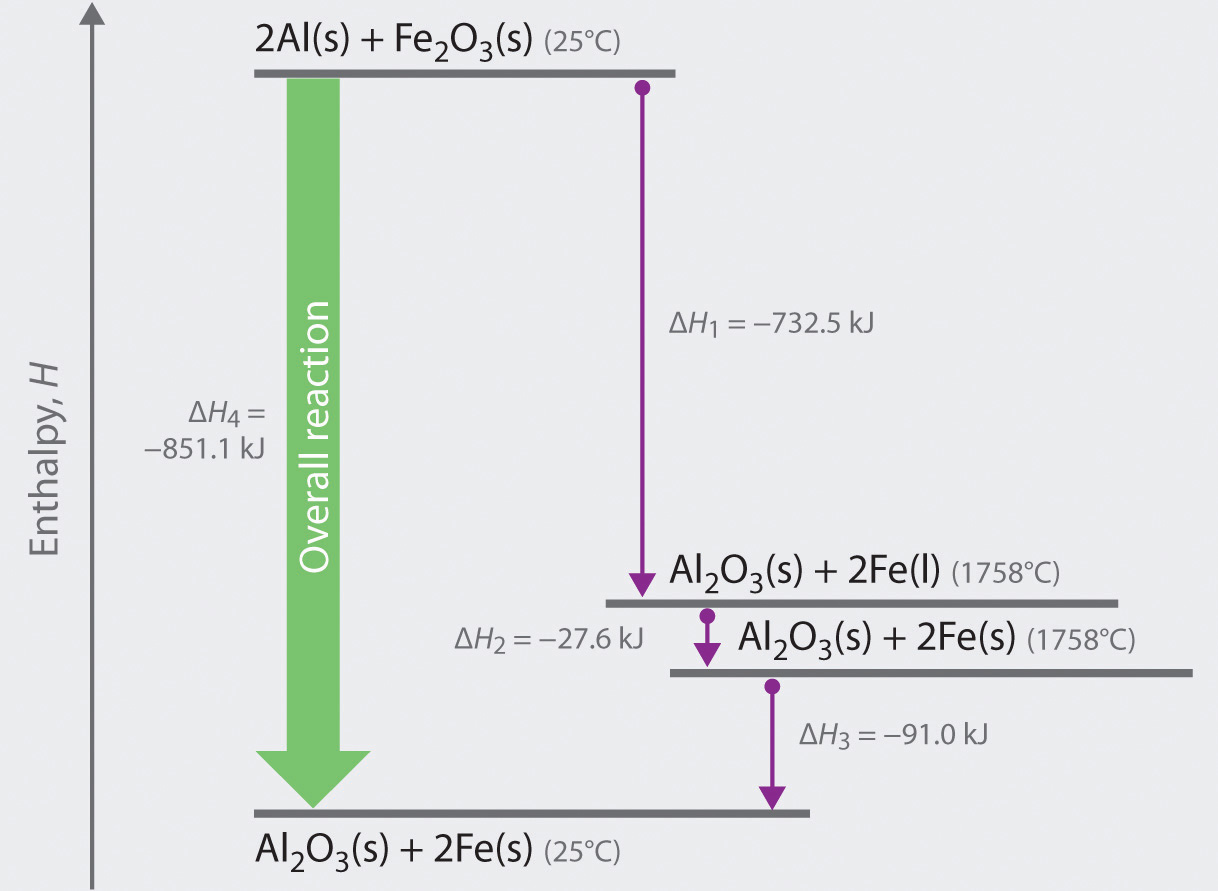


0 Response to "40 complete the enthalpy diagram for an ionic compound dissolving in water."
Post a Comment