40 Ammonia Molecular Orbital Diagram
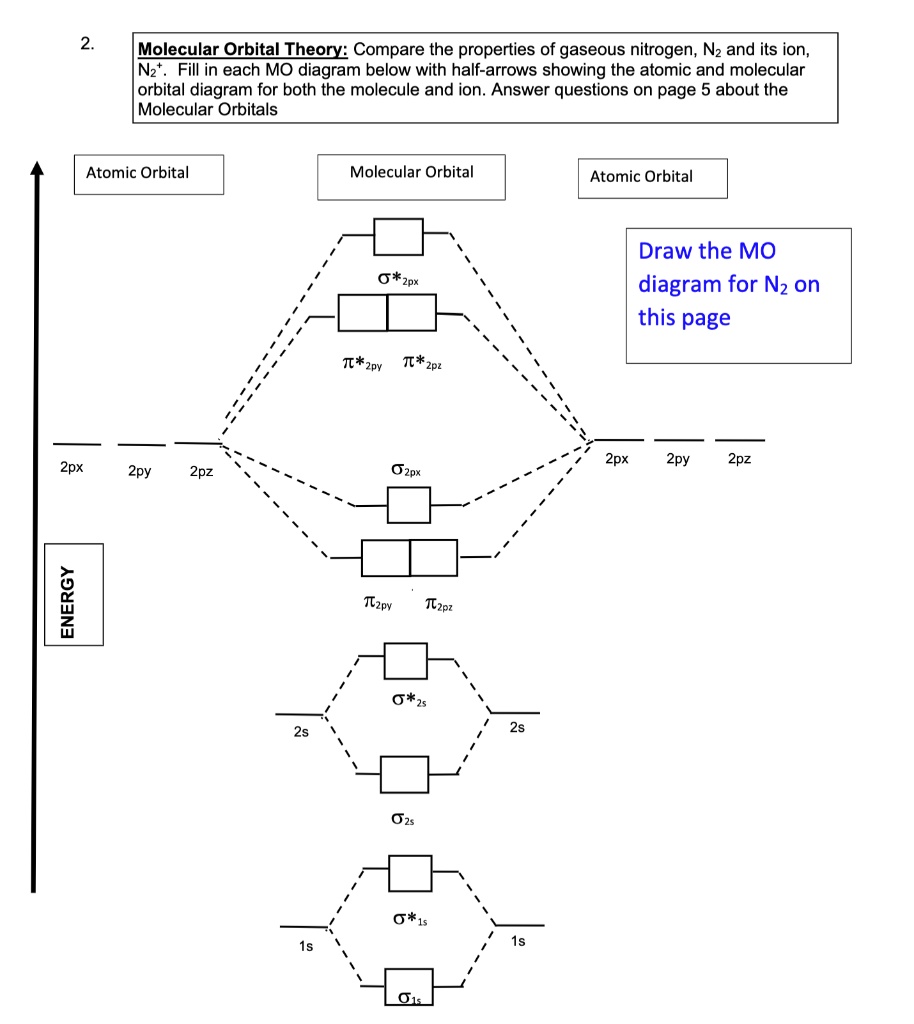
40 molecular orbital diagram for n2 - Wiring Diagram Source Molecular orbital diagram for n2. No2 Valence Electrons - 9 images - solved question 9 of 13 determine the number of valence e, ppt chemical bonding powerpoint presentation free, Shouldn't you count the valence electrons for Be (which is 2) and subtract 1 because of the + sign? For O2, N2, NO, F2, etc, you count the number of valence ... Ammonia Formula: Structure, Uses, Chemical Formula ({rm{N ... Molecular Geometry of Ammonia The \({\rm{s}}{{\rm{p}}^3}\) hybrid orbitals repel each other and are directed towards four corners of a regular tetrahedron. Hence the geometry should be tetrahedral, but the bond pairs suffer repulsion from the lone pair and distort the tetrahedral geometry to trigonal pyramidal or distorted tetrahedral structure.
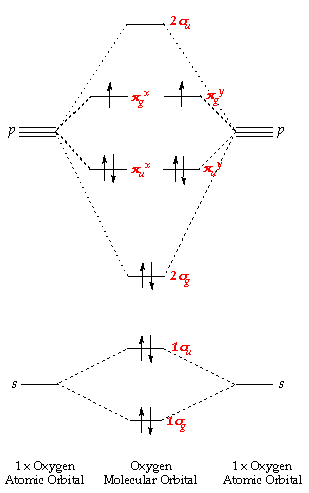
How To Draw Molecular Orbital Diagram Of Co - Drawing ... Molecular orbital diagram for oxygen gas (o2).fill from the bottom up, with 12 electrons total.bonding order is 2, and it is paramagnetic.sigma2s(2),sigma2s*. Building molecular orbital diagrams for homonuclear and heteronuclear diatomic molecules; (1) e n = 13.6 z e f f 2 n 2 e v.

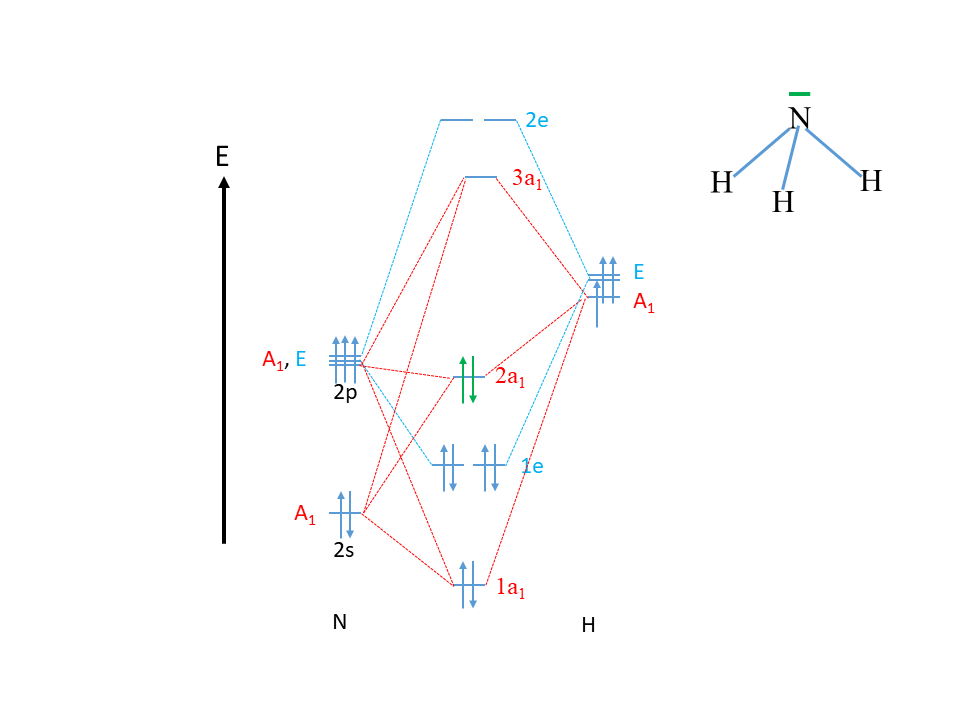
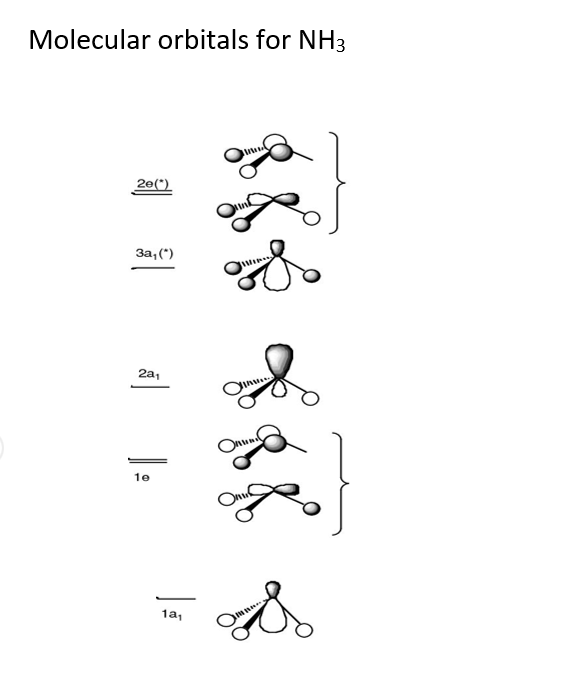
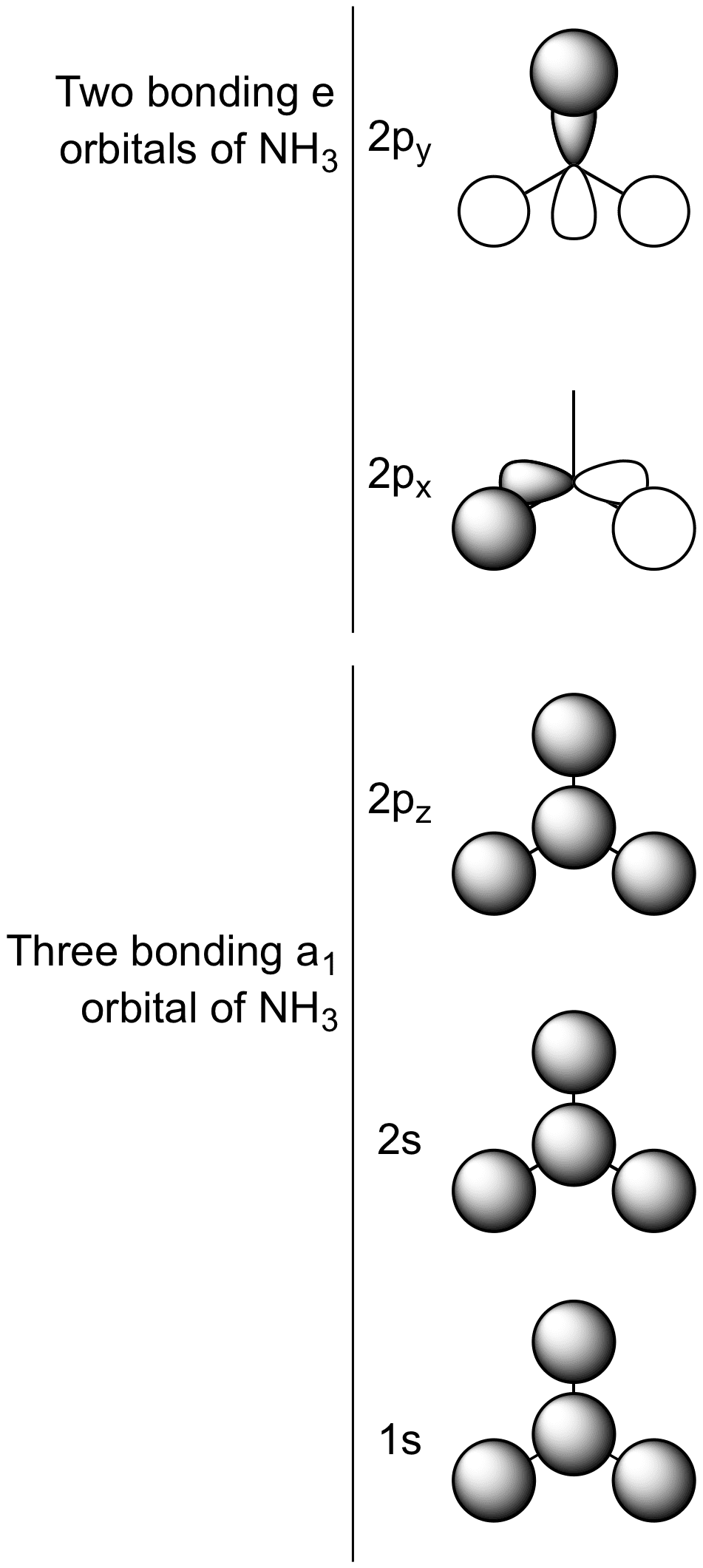
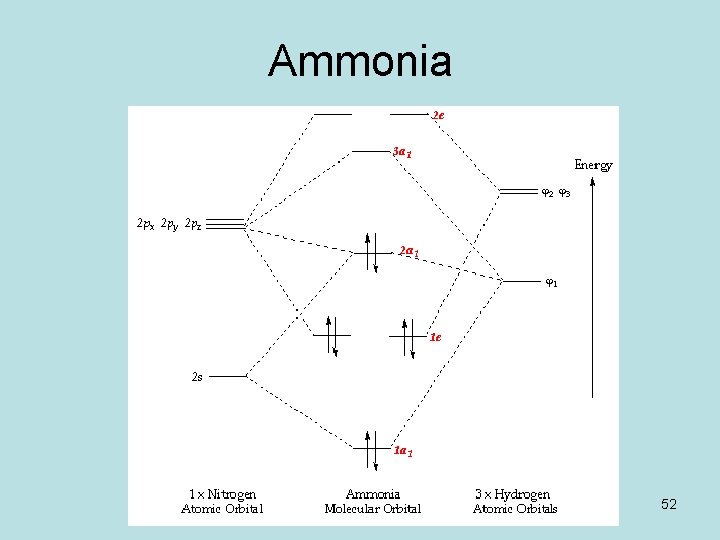
Ammonia molecular orbital diagram
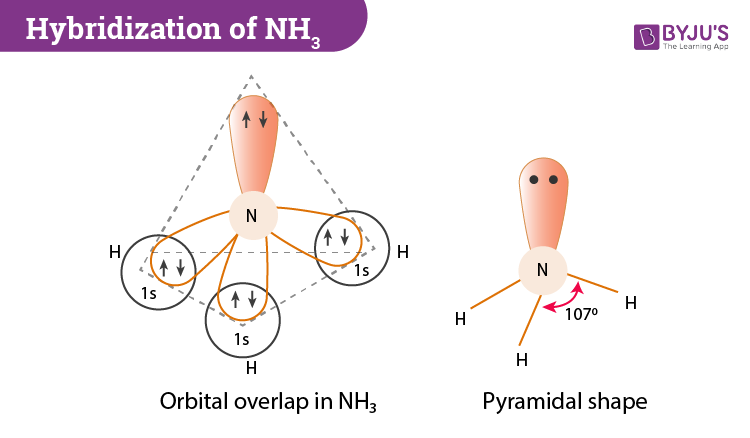
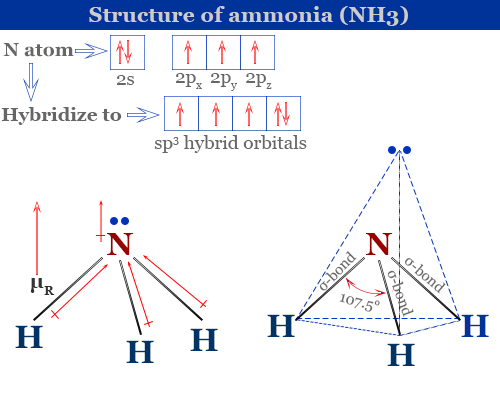
Ammonia Mo Diagram - molecular orbitals of the allyl anion ... salc ammonia molecular orbitals. Ammonia Mo Diagram. Here are a number of highest rated Ammonia Mo Diagram pictures upon internet. We identified it from obedient source. Its submitted by dealing out in the best field. We receive this kind of Ammonia Mo Diagram graphic could possibly be the most trending subject once we allowance it in google ... Orbital Shape And Hybridization Of Molecules Jan 21, 2022 · The diagram showing orbital overlapping in the ammonia (NH3) molecule. The orbitals of NH3 participating in the bond formation to undergo sp3 hybridization . Molecular orbital diagram of ammonia (NH3) molecule. The molecular orbital diagram is a diagrammatic representation of how chemical bonding is taking place within the ... Ammonia Formula: NH3 Equation, Formation, Structure - Embibe The nitrogen atom in ammonia is s p 3 hybridised [ 1 ( 2 s) + 3 ( 2 p) = 4 s p 3] This means the atomic orbitals of the nitrogen atom undergo intermixing to form 4 s p 3 hybridised orbitals. The 3 half-filled s p 3 hybrid orbitals of Nitrogen overlap with three 1 s orbitals of hydrogen atoms to form sigma bonds.
Ammonia molecular orbital diagram. Why is ammonia not formed of unhybridised (pure) orbitals ... Answer: Answer is simple. Plz.see if N atom uses its 3 half-filled orbitals ( we can call them pure p - orbitals) then bond angle in NH3 should be 90°. However bond angle in NH3 is 107° , so in order to explain its bond angle participation of s- orbital contribution is needed. Hence concept of ... NH3 Lewis Structure, Molecular Geometry, Hybridization ... Ammonia is a stable binary hydride having a Trigonal Pyramidal molecular geometry and sp3 hybridization. It has bond angles of 107 degrees and an electron geometry of tetrahedral. To know more about its polarity, read our blog on polarity. About Priyanka To read, write and know something new every day is the only way I see my day! 44 write the orbital diagram of carbon before sp3 ... The carbon atom in methane exhibits sp3 hybridization. We illustrate the orbitals and electron distribution in an isolated carbon atom and in the bonded atom in CH4 in Figure 11. March 2, 2021 - 2. Draw the energy diagram for the orbitals of sp3 hybridzied carbon and nitrogen. Then fill in the correct number of electron. 3. Nh3 Lewis Structure Molecular Geometry - ViralListClub.com The Lewis structure of ammonia NH3 would be three hydrogen atoms bonded to a nitrogen atom in the middle with a lone pair of electrons on top of the atom. Due to the presence of two lone pairs of electrons that repel bond pairs N-H it acquires a bent V-shape molecular shape with a bond angle of 1045.
Is NH3 Acid or Base?| NH3 Properties - What's Insight NH3 Molecular Geomoetry. Ammonia (NH3) has a trigonal pyramidal or distorted tetrahedral molecular shape. It is due to the presence of a single non-bonding lone pair of electrons on the nitrogen atom, which acts as a repulsive force on the bonding orbitals.. In the centre of the NH3 molecular geometry, three hydrogen atoms are connected to a nitrogen atom. › definition-of-molecular-weightWhat Is Molecular Weight? Chemistry Definition Jul 03, 2019 · Molecular Weight Versus Molecular Mass . Molecular weight is often used interchangeably with molecular mass in chemistry, although technically there is a difference between the two. Molecular mass is a measure of mass and molecular weight is a measure of force acting on the molecular mass. A more correct term for both molecular weight and ... Study of the structural features and solvent effects using ... Molecular dynamics of atogepant with solvents like water and ammonia did using ORCA 4.0 software with simple point calculation timestep 0.5 femtosecond (fs), Initvel 350 K, and time of 10.0 fs . Fig. 5 shows the drift of atogepant with water and ammonia in K versus time, and Fig.6 shows the simulations of atogepant with water and ammonia by ... › 2020 › 11NH2- Lewis Structure, Molecular Geometry, Polarity ... Nov 10, 2020 · Steps to be followed for drawing NH2- Lewis structure. 1. Find out the total number of valence electrons. Here the amide ion is made up of two different atoms: Nitrogen (N) and Hydrogen (H) so first, we have to figure out the valence electrons of these two atoms separately.
What Is The Electron Domain Geometry Of Nh3? - SupportMyMoto Formation of Ammonia Molecule ( NH3): Want of Hybridization in Ammonia Molecule: In nitrogen atom, there are three half-filled 2p orbitals and the valency needs to be 3 and it's three. These three 'p' orbitals are perpendicular to one another and in the event that they kind bonds, the angle between them needs to be 90°. NH3 Molecular Geometry| Trigonal Pyramidal - What's Insight Ammonia (NH3) has a trigonal pyramidal or distorted tetrahedral molecular shape. It is due to the presence of a single non-bonding lone pair of electrons on the nitrogen atom, which acts as a repulsive force on the bonding orbitals. In NH3 molecular geometry, three hydrogen atoms are bonded to a nitrogen atom in the middle. Ethene vs. ammonia - CHEMISTRY COMMUNITY Ethene vs. ammonia. Postby Ella Bogomilsky 2B » Mon Nov 15, 2021 3:09 am. In ammonia, the 5 valence electrons on nitrogen all go into the hybridized orbitals. In ethene, the 4 valence electrons on carbon go into the hybridized orbitals, and one goes into the unhybridized p orbital. Why does this happen? What is the electron geometry and molecular geometry of ... NH3 Hybridization The Nitrogen atom has the electronic configuration of 1s2 2s2 2px1 2py1 2pz1. When it shares the electrons with Hydrogen atoms, one s-orbital and three p-orbitals hybridize and overlaps with s orbitals of a Hydrogen atom to form sp3 hybridization. Thus, Ammonia or NH3 has sp3 hybridization.
Trigonal Pyramidal | Geometry of Ammonia | UO Chemists The molecular formula of ammonia is NH 3. The central atom is nitrogen having atomic number 7. The electronic configuration of nitrogen is 1s 2, 2s 2, 2p x1 2p y1 2p z1. The hydrogen atomic number is 1. Its electronic configuration is 1s 1. If 1s orbitals of hydrogen atoms overlap with three p orbitals of one nitrogen atom.
Dinitrogen Fixation: Rationalizing Strategies Utilizing ... Simple molecular orbitals models are a convenient way to simply illustrate and understand structural details and reactivity, and complementary to more in‐depth computational studies. [22] The free dinitrogen molecular orbital diagram is shown in Scheme 2. Most mononuclear d‐block metal complexes coordinate N 2 in an end‐on fashion.
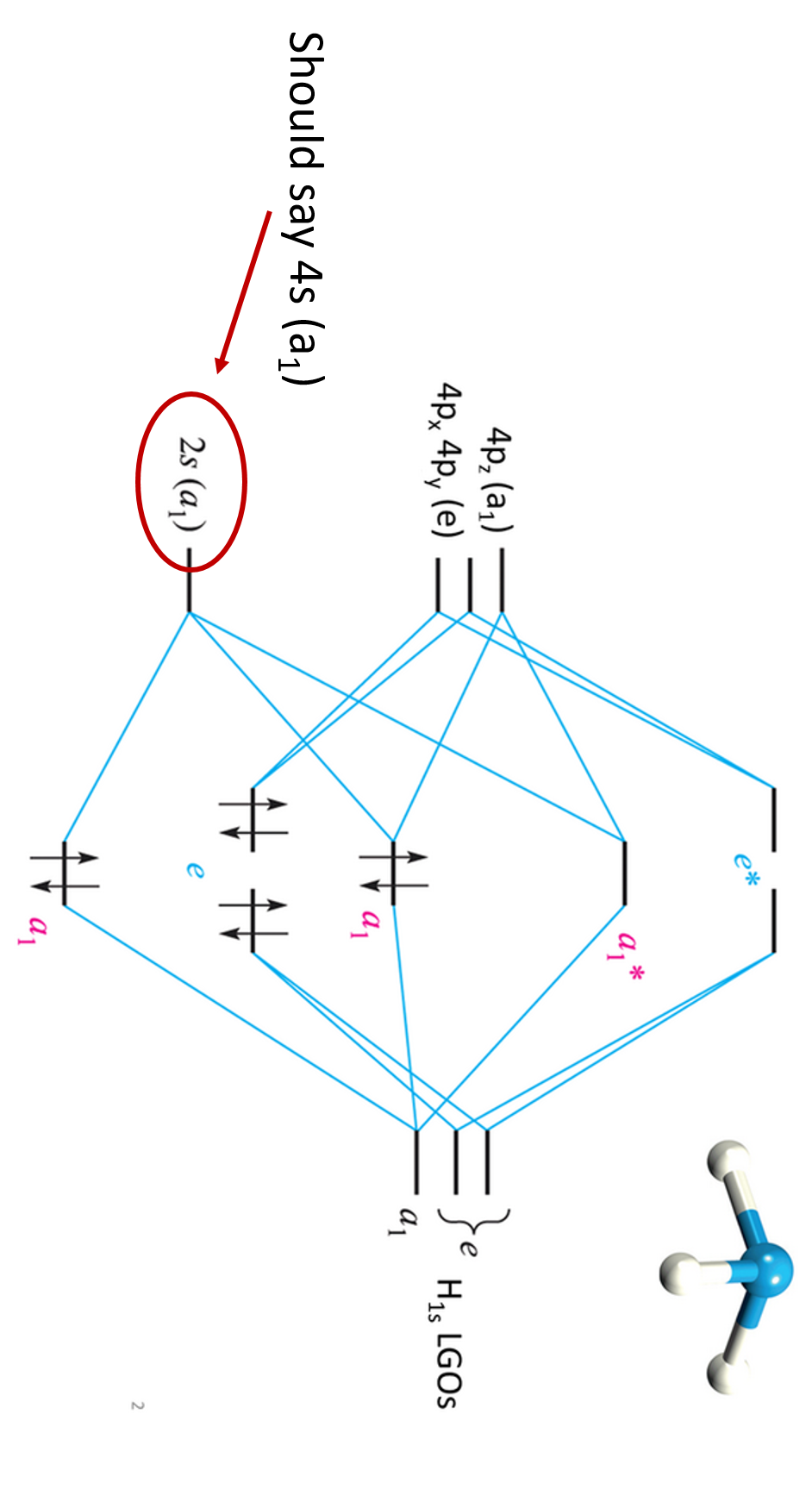
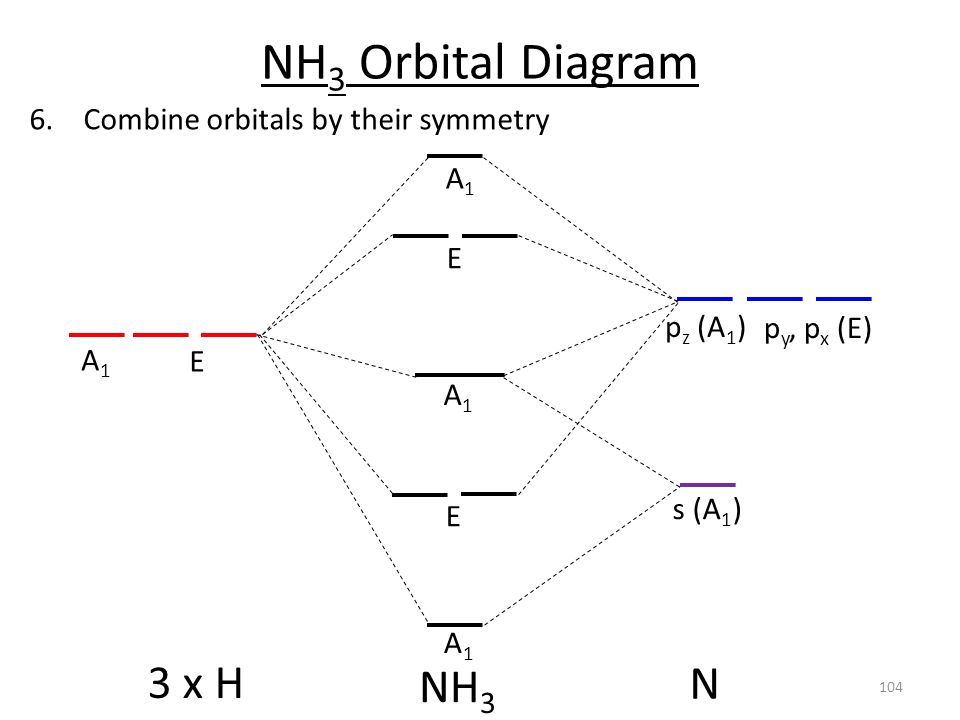
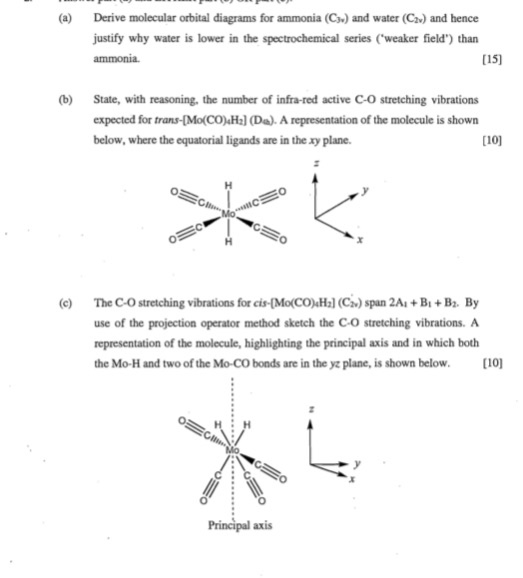
3.5.4: NH₃ - Chemistry LibreTexts We will walk through the steps below to construct the molecular orbital diagram of ammonia. Preliminary Steps Step 1. Find the point group of the molecule and assign Cartesian coordinates so that z is the principle axis. The NH 3 molecule is trigonal pyramidal and its point group is C3v. The z axis is collinear with principle axis, the C3 axis.
› articles › s41557/021/00852-6A thiolate-bridged FeIVFeIV μ-nitrido complex and its ... Dec 23, 2021 · The molecular structure of complex 3[BPh 4] was fully characterized by X-ray diffraction analysis (Fig. 2b).Unlike most diiron µ-nitrides 10,11,12,14, 3[BPh 4] consists of a bent Fe–N–Fe ...
29 Cn Molecular Orbital Diagram Wiring Diagram List ... Molecular orbital diagram this is a molecular orbital energy level diagram for the p orbitals. note that the σ bonding orbital is lowest in energy due to the greater overlap end onend. σu πg 2p πu σg 2p 29. molecular orbital diagram the alternate notation is provided on the right side of the energy level diagram. Pig skeleton diagram xcode ...
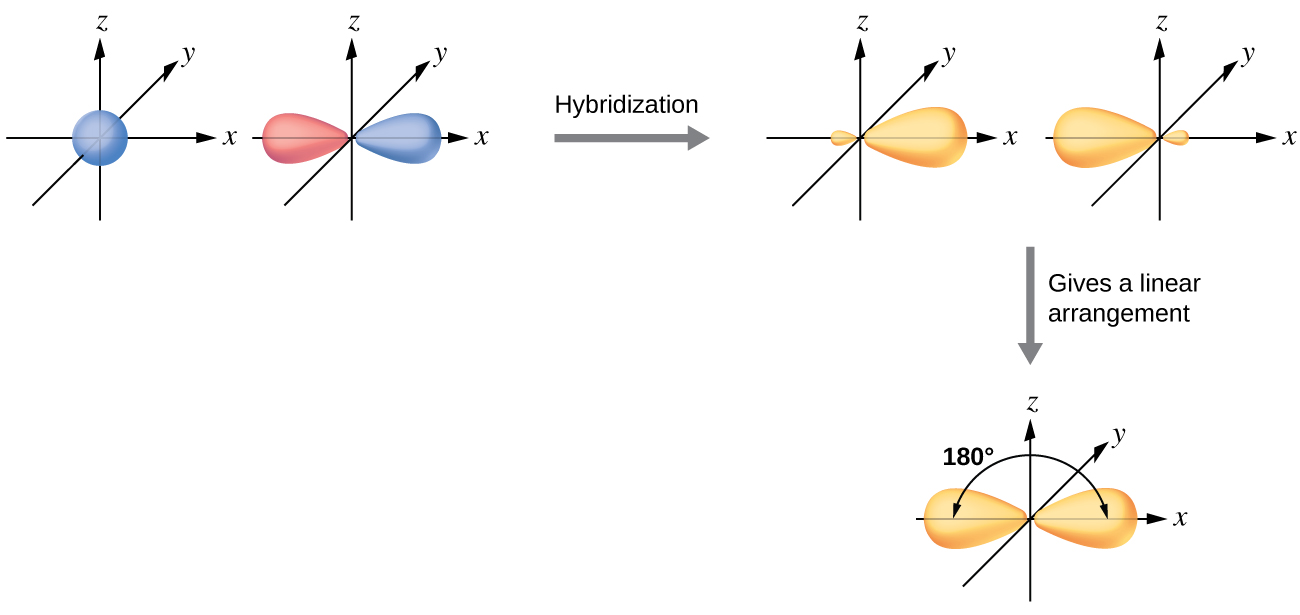
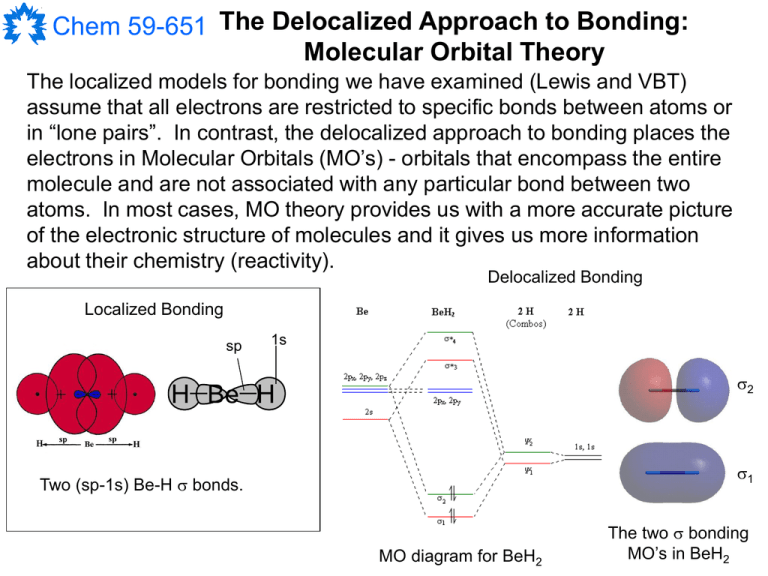
41 polyatomic molecular orbital diagram A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular.Chemistry: The Central Science, Chapter 9, Section 5Chapter 9 - Molecular Geometry
topblogtenz.com › hclo3-lewis-structure-molecularHClO3 lewis structure, Molecular geometry, Acid or Base ... First of all, determine the valence electron that is available for drawing the lewis structure of HClO3 because the lewis diagram is all about the representation of valence electrons on atoms. So, an easy way to find the valence electron of atoms in the HClO3 molecule is, just to look at the periodic group of hydrogen, oxygen, and chlorine atoms.
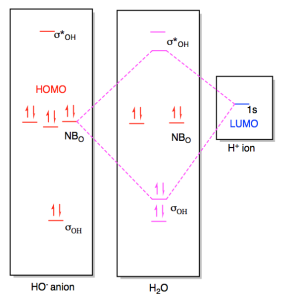
www1.lasalle.edu › ~prushan › IC-articlesPolyatomic Molecular Orbital Theory - La Salle University Molecular Orbital Theory – Walsh diagram The Walsh diagram shows what happens to the molecular orbitals for a set of molecules which are related in structure. In this case, the difference is the H-X-H bond angle which decreases from 180 o to 90 o Molecular Orbital Theory – Walsh diagram Water 104.5 ° X H H H O H
techiescientist.com › nh4-lewis-structureNH4+ Lewis Structure, Molecular Geometry, and Hybridization Feb 17, 2022 · Conclusion. The concepts of Lewis Structure, Molecular Geometry, and Hybridization hold great significance in understanding the structure, geometry, and subsequently the behavior of a substance, which is a direct result of the properties of associated element’s atoms.
9.7: Molecular Orbitals - Chemistry LibreTexts Figure 9.7. 3: Molecular Orbital Energy-Level Diagrams for Diatomic Molecules with Only 1 s Atomic Orbitals. (a) The H 2+ ion, (b) the He 2+ ion, and (c) the He 2 molecule are shown here. Figure 9.7. 3 a shows the energy-level diagram for the H 2+ ion, which contains two protons and only one electron.
Why does ammonia have a tetrahedral electron geometry but ... Answer: Because we describe MOLECULAR geometry on the basis of the distribution of ATOMS, not electronic pairs… Granted, electronic geometry is tetrahedral, but the nitrogen, and hydrogen atoms are arranged in a pyramid … and ∠_{H-N-H} \approx 105°, i.e. down from the tetrahedral angle of 109.5°....
NCERT Notes For Class 11 Chemistry Chapter 4 Chemical ... iii) Formation of Ammonia (NH 3) molecule. In NH 3, the central atom N has the electronic configuration 1s 2 2s 2 2p 3. The one s-orbital and three p-orbitals of N undergo sp 3 hybridisation to form 4 sp 3 hybrid orbitals. ... Formation of molecular orbitals - Linear Combination of Atomic Orbitals (LCAO) method.
Construct The Molecular Orbital Diagram For H2 And Then ... A molecular orbital diagram is used to define chemical bonding in a molecule. This chart is based ~ above the molecular orbital theory. According to the molecular orbital theory, molecule orbitals the a molecule is created by combination of its atomic orbitals and electrons room distributed among the molecule orbitals.
Ammonia Molecule Diagram - gcse chemistry covalent bonding ... Ammonia Molecule Diagram. Here are a number of highest rated Ammonia Molecule Diagram pictures on internet. We identified it from trustworthy source. Its submitted by organization in the best field. We say yes this kind of Ammonia Molecule Diagram graphic could possibly be the most trending subject behind we part it in google plus or facebook.
Nh3 Mo Diagram - symmetry cisplatin c2v, nh3 tpd profile ... H2O Molecular Orbital Diagram. NH3 Lewis Dot Diagram. Ammonia Diagram. BH3 MO Diagram. MO Diagram Of No. NH3 Hybridization. CN Molecular Orbital Diagram. Orbital Energy Level Diagram. N2 2 Molecular Orbital Diagram. Molecular Orbital Of Co. NH3 Molecule. CH2 MO Diagram.
techiescientist.com › hno3-lewis-structureHNO3 Lewis Structure, Molecular Geometry ... - Techiescientist Feb 17, 2022 · MO Diagram of HNO3. The sigma bonds between N and O atoms are formed by 2sp2 orbital of nitrogen and a hybrid orbital atom from O atoms. As a result, three sigma bonding and antibonding orbitals are formed. The single sigma bond between hydrogen and oxygen use 1s orbital of hydrogen 2sp3 orbitals of oxygen.
Ammonia: Molecular Geometry - Hybridization - Molecular ... The hybrid orbitals are more prominent outward so that their ability to overlap is stronger than that of normal orbitals. Molecular Formula: A chemical formula is a brief way of expressing the number and type of atoms that make up a particular chemical compound.
Chemical Bonding and Molecular Structure Class 11 ... In an anti-bonding molecular orbital, electron density is minimum. (a) around one atom of the molecule. (b) between the two nuclei of the molecule. (c) at the region away from the nuclei of the molecule. (d) at no place. Answer. B. Question. When two atomic orbitals combine, they form.
Ammonia Formula: NH3 Equation, Formation, Structure - Embibe The nitrogen atom in ammonia is s p 3 hybridised [ 1 ( 2 s) + 3 ( 2 p) = 4 s p 3] This means the atomic orbitals of the nitrogen atom undergo intermixing to form 4 s p 3 hybridised orbitals. The 3 half-filled s p 3 hybrid orbitals of Nitrogen overlap with three 1 s orbitals of hydrogen atoms to form sigma bonds.
Orbital Shape And Hybridization Of Molecules Jan 21, 2022 · The diagram showing orbital overlapping in the ammonia (NH3) molecule. The orbitals of NH3 participating in the bond formation to undergo sp3 hybridization . Molecular orbital diagram of ammonia (NH3) molecule. The molecular orbital diagram is a diagrammatic representation of how chemical bonding is taking place within the ...
Ammonia Mo Diagram - molecular orbitals of the allyl anion ... salc ammonia molecular orbitals. Ammonia Mo Diagram. Here are a number of highest rated Ammonia Mo Diagram pictures upon internet. We identified it from obedient source. Its submitted by dealing out in the best field. We receive this kind of Ammonia Mo Diagram graphic could possibly be the most trending subject once we allowance it in google ...





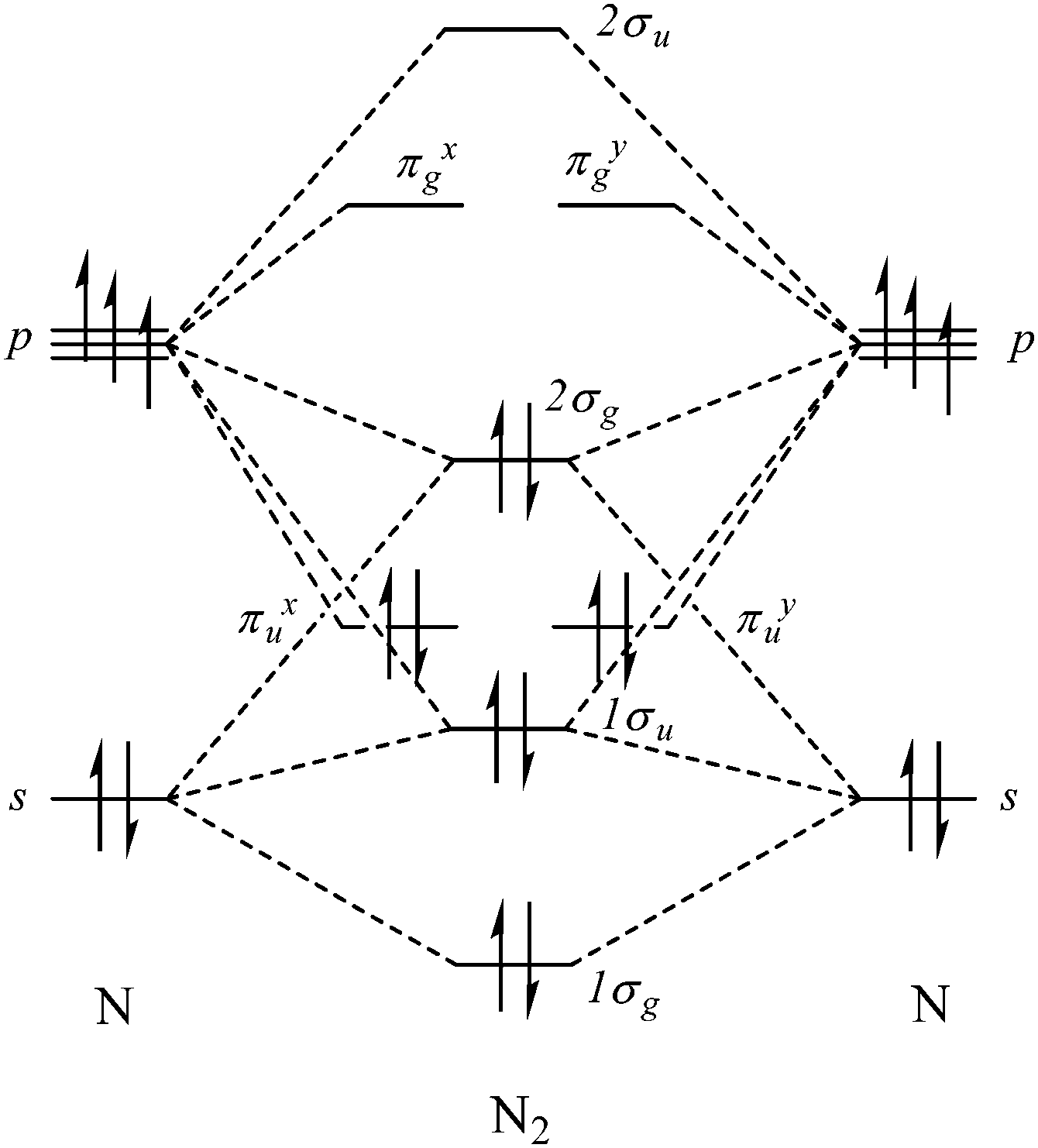












0 Response to "40 Ammonia Molecular Orbital Diagram"
Post a Comment