39 Carbon Dioxide Phase Diagram
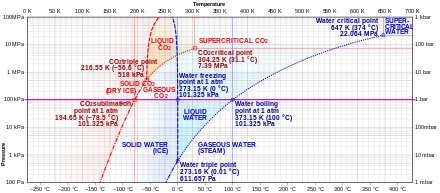
File:Carbon dioxide pressure-temperature phase diagram.svg ... File:Carbon dioxide pressure-temperature phase diagram.svg. Size of this PNG preview of this SVG file: 636 × 600 pixels. Other resolutions: 254 × 240 pixels | 509 × 480 pixels | 814 × 768 pixels | 1,085 × 1,024 pixels | 2,171 × 2,048 pixels | 742 × 700 pixels. Carbon dioxide - Wikipedia Carbon dioxide (chemical formula CO 2) is an acidic colorless gas with a density about 53% higher than that of dry air. Carbon dioxide molecules consist of a carbon atom covalently double bonded to two oxygen atoms. It occurs naturally in Earth's atmosphere as a trace gas.The current concentration is about 0.04% (412 ppm) by volume, having risen from pre-industrial levels of 280 ppm.
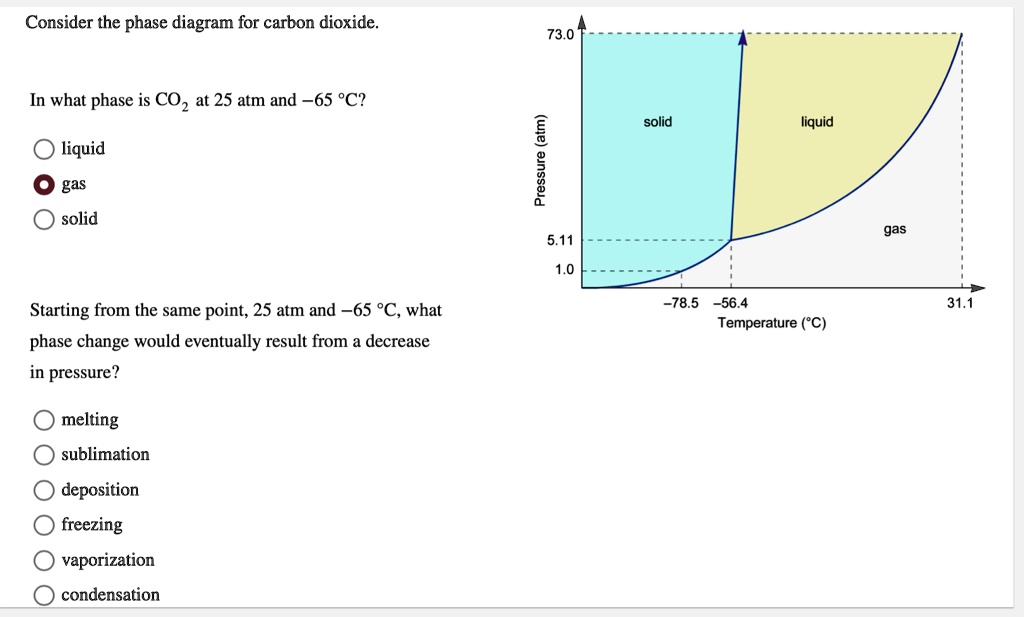
Phase Diagrams Flashcards | Quizlet Use the phase diagram for CO2 in the interactive activity and determine which of the following statements are correct. CO2 is a gas under normal conditions of temperature and pressure. All three phases of CO2 exist simultaneously at the triple point. When the pressure is 4 atm and the temperature is more than −56.7∘C, CO2 exists as a solid.

Carbon dioxide phase diagram
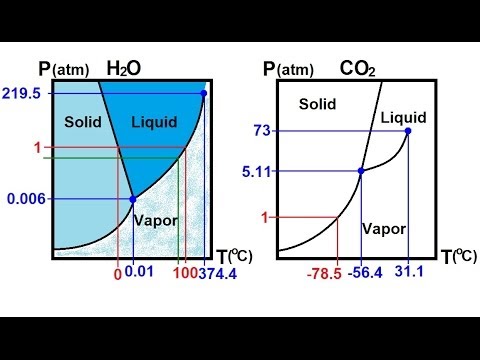
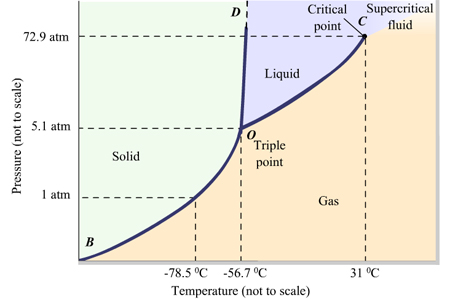
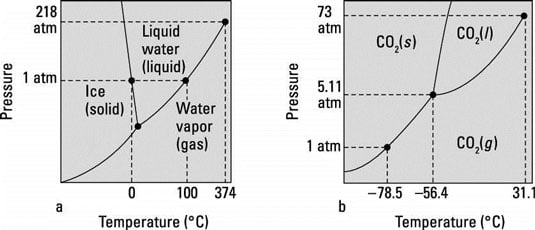
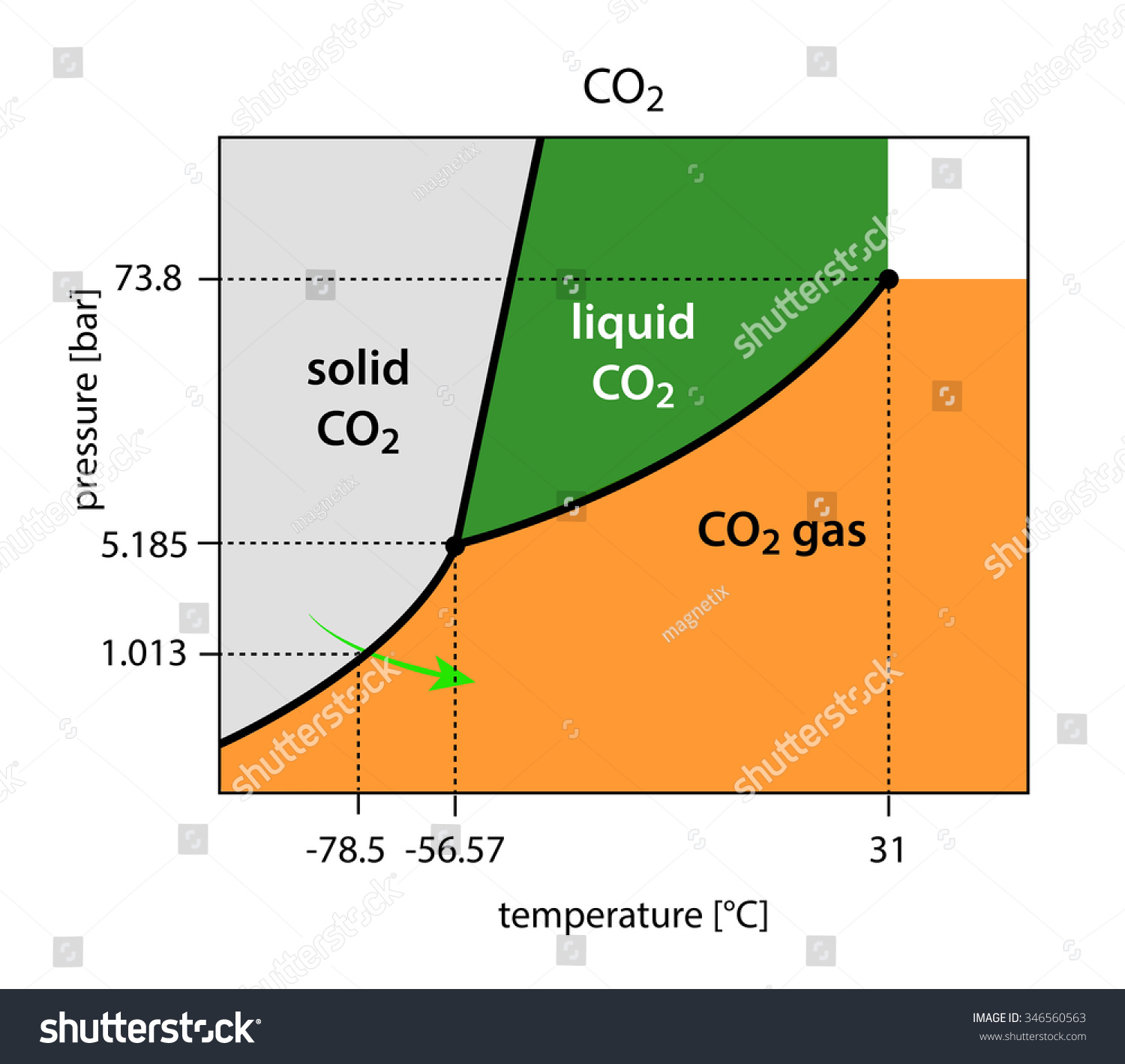
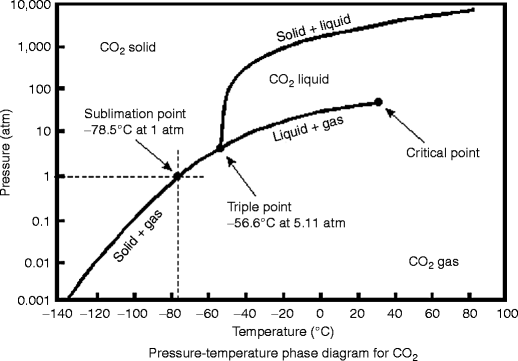
Phase Diagrams of Water & CO2 Explained - Chemistry ... This chemistry video tutorial explains the concepts behind the phase diagram of CO2 / Carbon Dioxide and the phase diagram of water / H2O. This video contai... Phase Diagrams: Carbon Dioxide and Water Phase Diagrams ... The phase diagram of water has a few notable differences from that of carbon dioxide. The fusion curve of carbon dioxide has a positive slope, while for water, the slope is negative. This is an atypical feature of water. Increasing the pressure favors a liquid-to- solid transition in carbon dioxide but a solid-to-liquid transition in water. phase diagrams of pure substances - chemguide The phase diagram for carbon dioxide The only thing special about this phase diagram is the position of the triple point which is well above atmospheric pressure. It is impossible to get any liquid carbon dioxide at pressures less than 5.11 atmospheres. That means that at 1 atmosphere pressure, carbon dioxide will sublime at a temperature of -78°C.
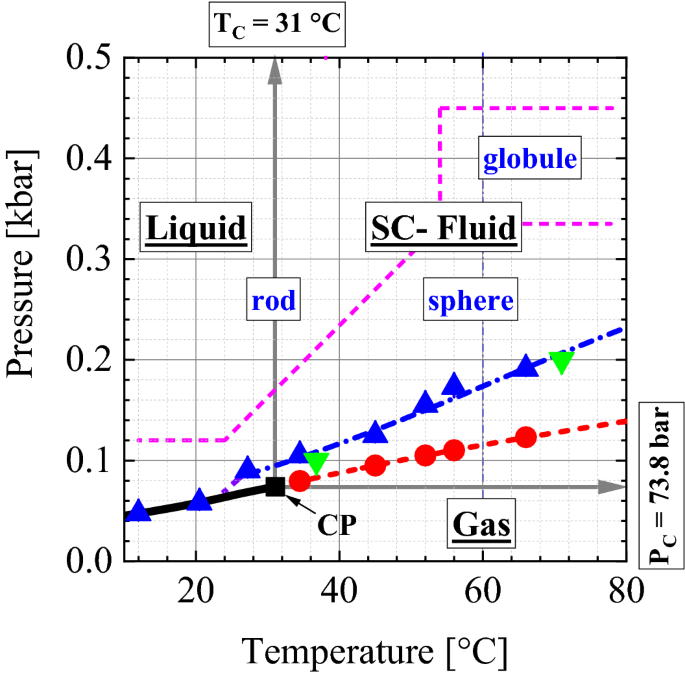
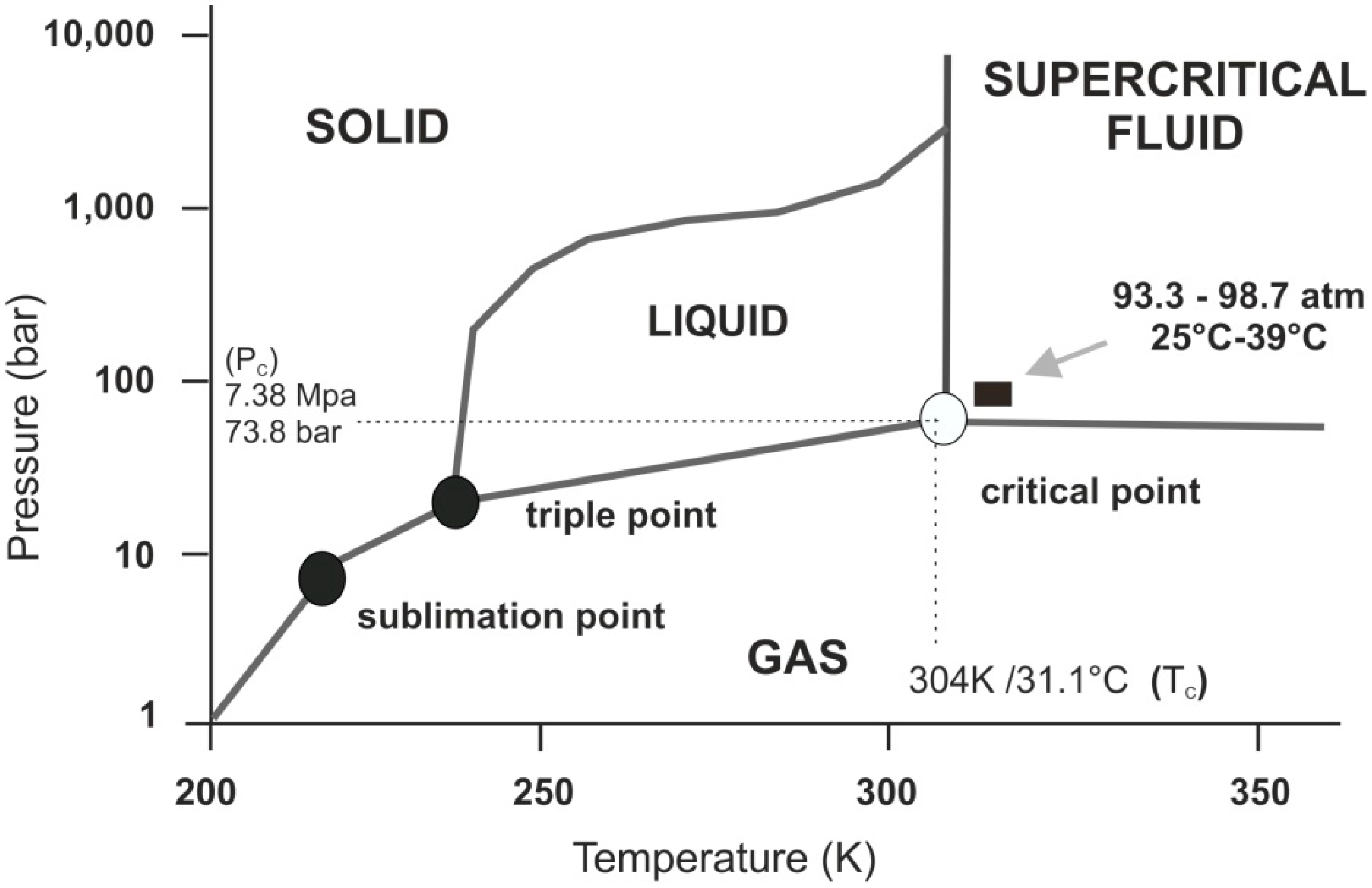
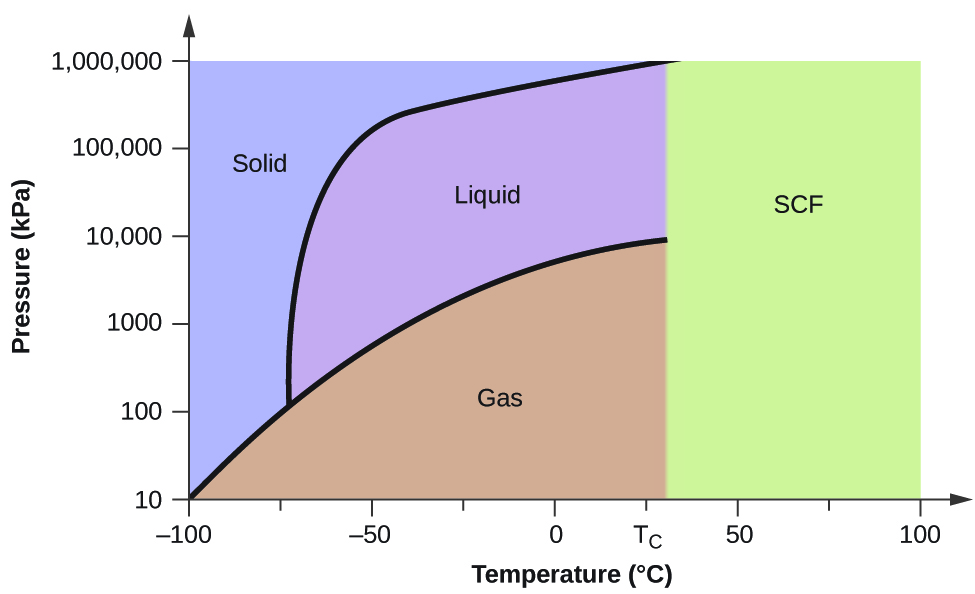
Carbon dioxide phase diagram. Carbon Dioxide - Thermophysical Properties The phase diagram for carbon dioxide shows the phase behavior with changes in temperature and pressure. The curve between the critical point and the triple point shows the carbon dioxide boiling point with changes in pressure. Phase diagram of carbon dioxide: evidence for a new ... A new phase (CO(2)-II) exists above 20 GPa and 500 K, which can be quenched to ambient temperature. The vibrational spectrum of this new CO(2) polymorph suggests the dimeric pairing of molecules. Based on the present in situ data and previous laser-heating results, we present new constraints for the phase diagram of carbon dioxide to 50 GPa and ... Phase Diagram of the Supercritical Carbon Dioxide ... The following reagents were used in the work: carbon dioxide of the first or highest grade with the CO 2 content of at least 99.5%, and also ethylcarbitol C 6 H 14 O 3 of the highest CAS 111-90- grade with the fraction of the main product of at least 99%.. The phase diagram was studied using the setup shown in Fig. 1 and described in detail in [3, 5-9] in accordance with the procedure ... Phase Diagrams - Chemistry - University of Hawaiʻi Determining the State of Carbon Dioxide Using the phase diagram for carbon dioxide shown in , determine the state of CO 2 at the following temperatures and pressures: (a) −30 °C and 2000 kPa (b) −60 °C and 1000 kPa (c) −60 °C and 100 kPa (d) 20 °C and 1500 kPa (e) 0 °C and 100 kPa (f) 20 °C and 100 kPa. Solution
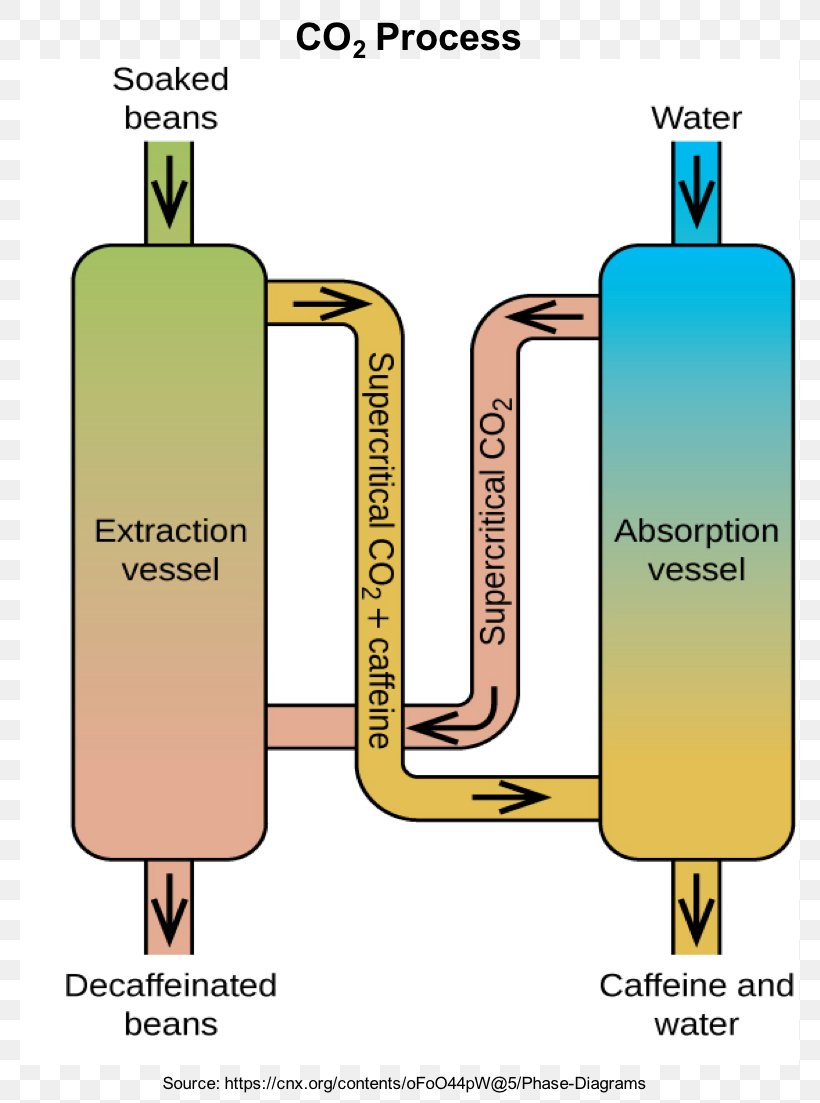
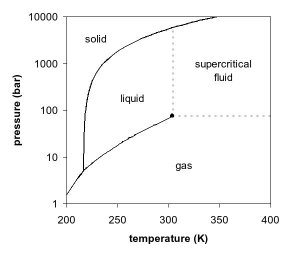
12.4: Phase Diagrams - Chemistry LibreTexts The Phase Diagram of Carbon Dioxide In contrast to the phase diagram of water, the phase diagram of CO 2 (Figure 12.4. 3) has a more typical melting curve, sloping up and to the right. The triple point is −56.6°C and 5.11 atm, which means that liquid CO 2 cannot exist at pressures lower than 5.11 atm. What Happens At The Triple Point Of Co2 ... Carbon Dioxide Phase Diagram In contrast to the phase diagram of water, the phase diagram of CO (Figure 12) shows the chemical composition of CO. The melting curve in 3) is more typical, with a slope to the right and a crest to the left. It is 56 degrees at the triple point. The temperature is 6C and 5F. CO2 Tables Calculator - Carbon Dioxide Properties Specific gas constant: R = 0.188923 kJ/ (kg·K) Specific gas constant: R = 0.0451235 Btu/ (lb·°F) 2. 1. Thermodynamic Properties - Main. Temperature. t. A phase diagram for CO2. | Download Scientific Diagram Carbon dioxide pressure-temperature phase diagram [33] The process starts from putting already cured solid polymers into an autoclave with supercritical fluid CO2 under the specific conditions ...
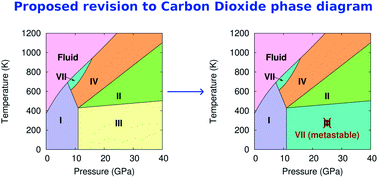
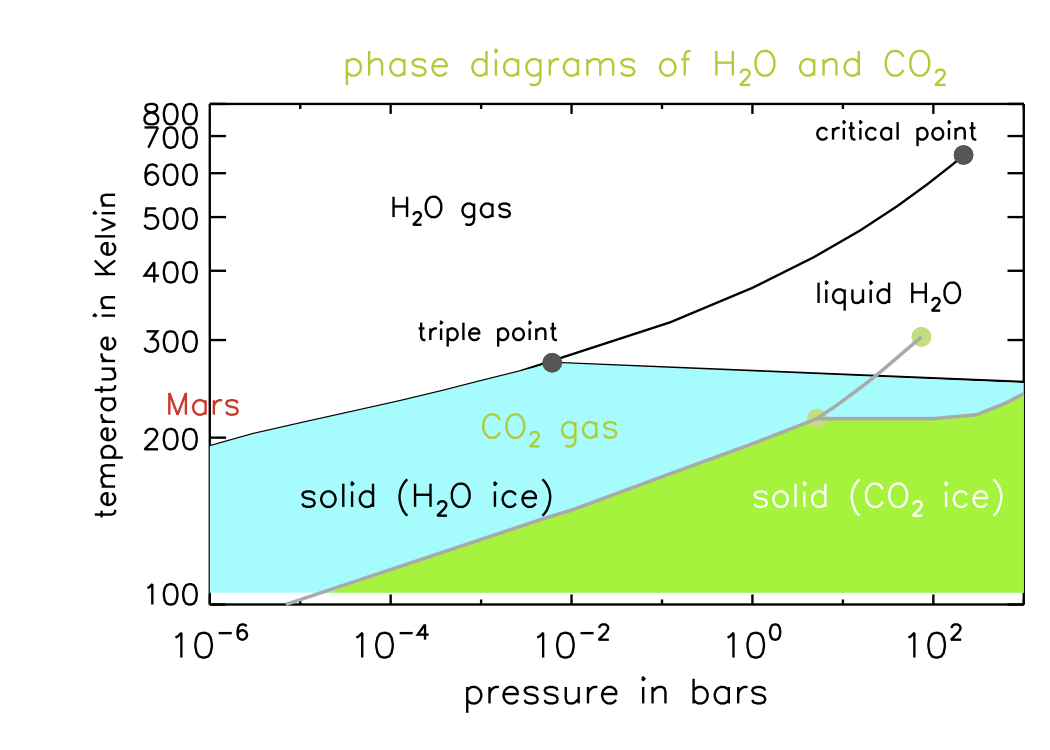
DOC Phase Diagram of Carbon Dioxide - University of Manitoba phase diagrams for carbon dioxide and water are shown in figure 1. A phase diagram shows the temperatures and pressures at which the various phases (i.e., solid, liquid and vapor) of a substance can exist. Both phase diagrams for water and carbon dioxide have the same general Y-shape , just shifted relative to one another. Carbon dioxide - NIST , The Densities of Coexisting Liquid and Gaseous Carbon Dioxide and the Solubility of Water in Liquid Carbon Dioxide., J. Am. Chem. Soc., 1927, 49, 2729-2734. [ all data ] Stephenson and Malanowski, 1987 Consider this phase diagram for carbon dio... | Clutch Prep Problem Details. Consider this phase diagram for carbon dioxide. In what phase is CO 2 at 72 atm and 0 °C? a) solid. b) liquid. c) gas. Starting from the point described above, what phase change would eventually result from a decrease in pressure? a) condensation. b) deposition. Phase diagram of carbon dioxide: Update and challenges ... Solid carbon dioxide exhibits a rich phase diagram at high pressures. Metastable phase III is formed by compressing dry ice above ∼10-12 GPa. Phase VII occurs at similar pressures but higher ...
10.4 Phase Diagrams - Chemistry Consider the phase diagram for carbon dioxide shown in Figure 5 as another example. The solid-liquid curve exhibits a positive slope, indicating that the melting point for CO 2 increases with pressure as it does for most substances (water being a notable exception as described previously). Notice that the triple point is well above 1 atm, indicating that carbon dioxide cannot exist as a liquid ...
Solved Directions: Analyze the phase diagram of water and ... Transcribed image text: Directions: Analyze the phase diagram of water and carbon dioxide. You may refer to your book for some information about the two phases and answer the questions that follow. 210 Sold Pre 0.000 273 2722 373 4 (a) Phase Diagram of Water (b) Phase Diagram of CO2 Processing Questions: Phase Diagram of Water "What can you say about the Phase Diagram of Water?
PDF Carbon Dioxide: Temperature - Pressure Diagram Carbon Dioxide: Temperature - Pressure Diagram S a t u r at i o n Li ne. Title: phase_diagram.xls Created Date: 11/10/1999 5:44:57 PM
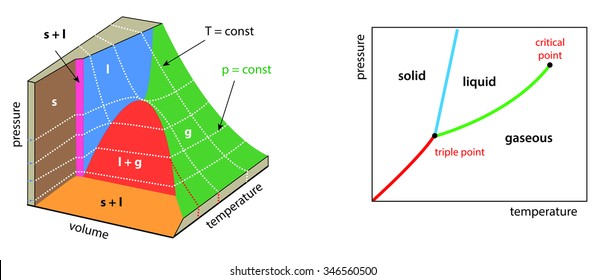
Is there any relevance between phase diagram and energy? Phase Diagram for Carbon Dioxide. I watched two diagrams; water and carbon dioxide. the difference between water and carbon dioxide is the gradient of the melting curve. The gradient of water is minus and the gradient of carbon dioxide is plus. Almost materials have plus, but I don't know why they have. So I thought of some possible things.
Phase Diagram | Explanation, Definition, Summary & Facts CO2 phase diagram The phase diagram of CO is more normal than phase diagram of water, as it's solid and liquid phase boundary tend to bend right side which means that increase in temperature and pressure leads to the conversion of solid phase into liquid phase.
Supercritical carbon dioxide - Wikipedia Carbon dioxide pressure-temperature phase diagram Supercritical carbon dioxide ( sCO 2) is a fluid state of carbon dioxide where it is held at or above its critical temperature and critical pressure .
Phase Diagrams - faculty.chem.queensu.ca Carbon dioxide phase diagram: Look at the features of carbon dioxide's phase diagram. the triple point occurs at a pressure of about 5.11 atm. So we cannot find liquid CO 2 under normal conditions; only solid and vapour.
Carbon dioxide | CO2 - PubChem Carbon dioxide is a one-carbon compound with formula CO2 in which the carbon is attached to each oxygen atom by a double bond.A colourless, odourless gas under normal conditions, it is produced during respiration by all animals, fungi and microorganisms that depend directly or indirectly on living or decaying plants for food.
What are the phase diagrams of water and carbon dioxide ... What are the phase diagrams of water and carbon dioxide? Chemistry Phases of Matter Phase Diagrams. 1 Answer Al E. Nov 23, 2017 Each line represents phase changes between respective boundaries. The triple point is where all the phases are at equilibrium. Phase changes are usually isothermal, so temperature is constant until every molecule has ...
The phase diagram of carbon dioxide from correlation ... The phase stability and equilibria of carbon dioxide are investigated from 125-325 K and 1-10 000 atm using extensive molecular dynamics (MD) simulations and the Two-Phase Thermodynamics (2PT) method. We devise a direct approach for calculating phase diagrams, in general, by considering the separate …
Predicting the phase diagram of solid carbon dioxide at ... Phase diagram of carbon dioxide. The red dots and blue line are the calculated transition boundary between phases II-III and phases I-III, respectively. The experimental data are taken from...
phase diagrams of pure substances - chemguide The phase diagram for carbon dioxide The only thing special about this phase diagram is the position of the triple point which is well above atmospheric pressure. It is impossible to get any liquid carbon dioxide at pressures less than 5.11 atmospheres. That means that at 1 atmosphere pressure, carbon dioxide will sublime at a temperature of -78°C.
Phase Diagrams: Carbon Dioxide and Water Phase Diagrams ... The phase diagram of water has a few notable differences from that of carbon dioxide. The fusion curve of carbon dioxide has a positive slope, while for water, the slope is negative. This is an atypical feature of water. Increasing the pressure favors a liquid-to- solid transition in carbon dioxide but a solid-to-liquid transition in water.
Phase Diagrams of Water & CO2 Explained - Chemistry ... This chemistry video tutorial explains the concepts behind the phase diagram of CO2 / Carbon Dioxide and the phase diagram of water / H2O. This video contai...














0 Response to "39 Carbon Dioxide Phase Diagram"
Post a Comment