38 vapour liquid equilibrium diagram
Vapour/liquid equilibrium is the state of coexistence of liquid and vapour phases. In the qualitative discussion, we shall focus on systems comprised of two chemical species, because systems of greater complexity cannot be adequately represented graphically. When N = 2 the phase rule becomes F = 4 ˇ. Since there must be at least one Liquid nitrogen is an interesting and even entertaining substance to use for everything from science experiments to haute cuisine. However, can your average person buy liquid nitrogen without special permission? This guide will help you und...
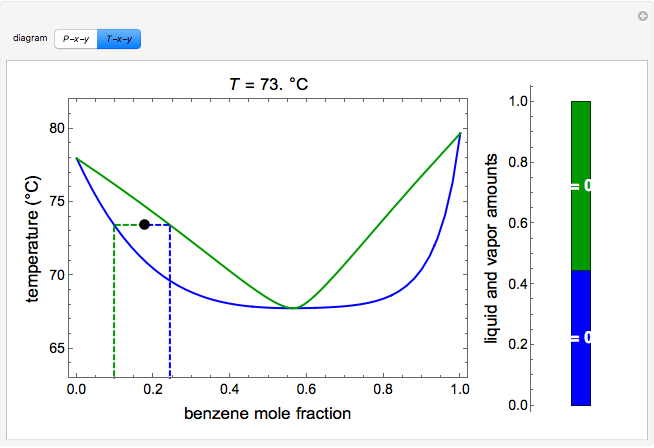
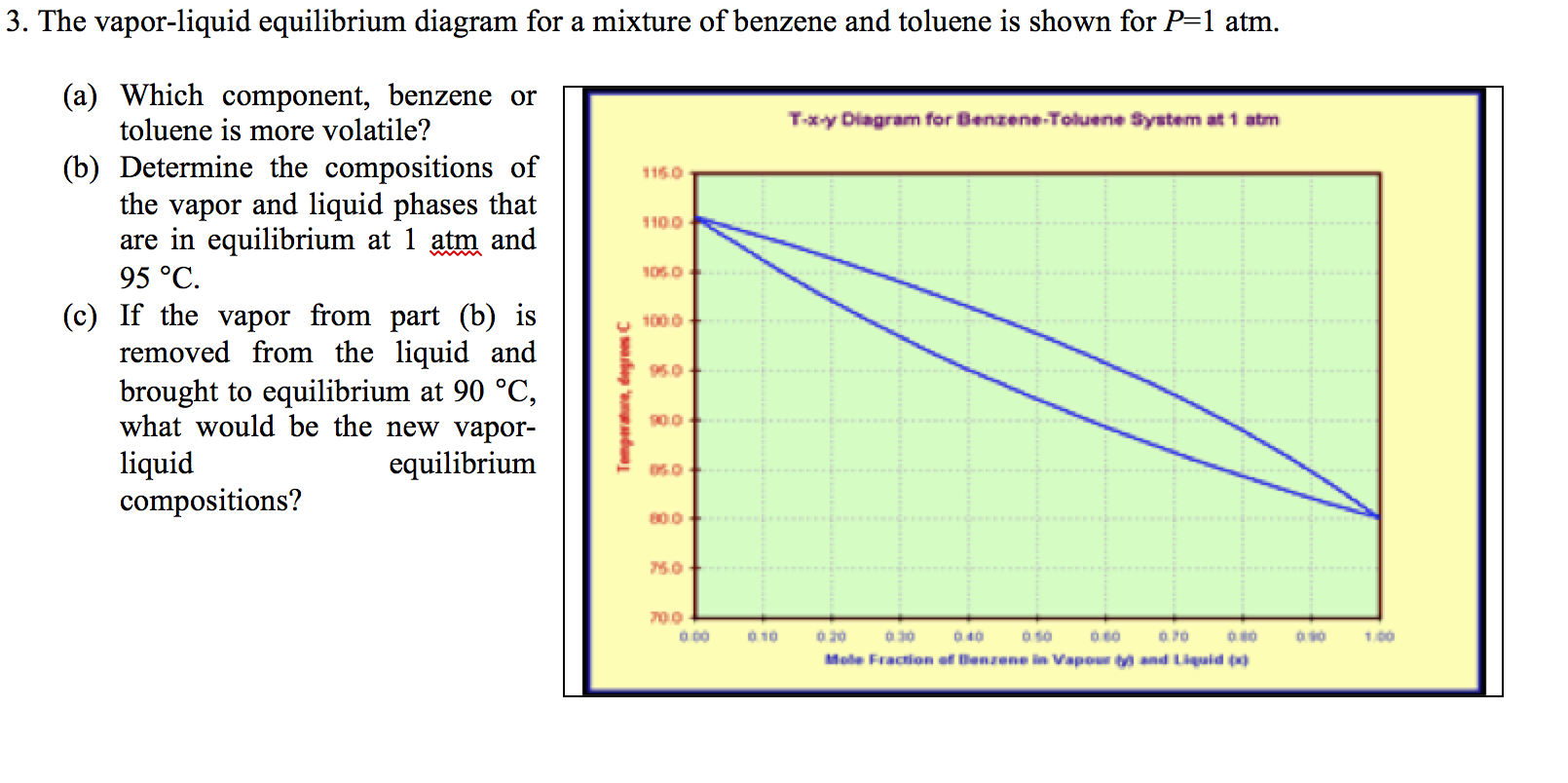
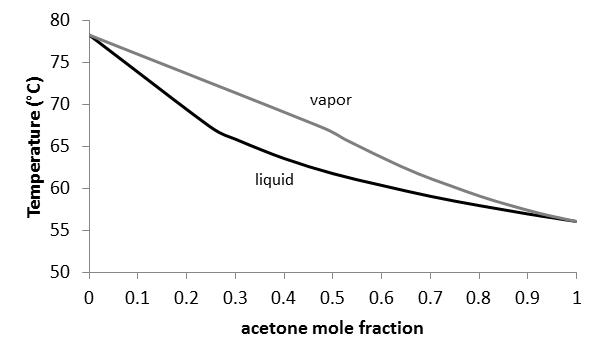
The vapor-liquid equilibrium relations for benzene (1)-toluene (2) at a total pressure of 200 kPa are given as a boiling-point Txy diagram shown in Figure 4.1-1. The upper curve is the saturated vapor curve (the dew-point curve) and the lower curve is the saturated liquid curve (the bubble-point curve).

Vapour liquid equilibrium diagram
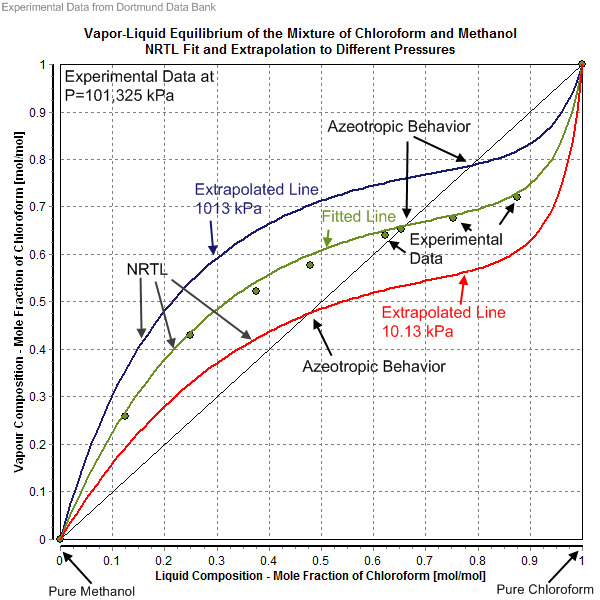
Vapor-Liquid Equilibrium Data. The experimental data shown in these pages are freely available and have been published already in the DDB Explorer Edition.The data represent a small sub list of all available data in the Dortmund Data Bank.For more data or any further information please search the DDB or contact DDBST.. Explorer Edition Data Main Page As is known, the general equation of state of van der Waals, published in the year 1873, as well as its numerous modifications, expresses the relation between pressure, volume and temperature of gases-vapours and liquids qualitatively well, but generally only with a rather rough approximation ... Calculation of vapor-liquid equilibrium (VLE) and drawing of phase diagrams. Name of substance. CAS-nr. Formula. Type of substance. acetone. 67-64-1. C 3 H 6 O. ketone.
Vapour liquid equilibrium diagram. In relation to the melting point, the triple point of a material occurs when the vapor, liquid, and solid phases are all in equilibrium (Table 4.10). This is the point on a phase diagram where the solid-vapor, solid-liquid, and liquid-vapor equilibrium lines all meet. JoVE publishes peer-reviewed scientific video protocols to accelerate biological, medical, chemical and physical research. Watch our scientific video articles. Vapour-liquid equilibrium diagrams Figure 5.1 presents the behaviour of a pure speciesthat can exist as solid, liquid or vapour in a pressure-temperature diagram. We may have three types of two-phase equilibrium solid/liquid, vapour/liquidand solid/vapour. There is a point where all three phases coexist, designated by the triple point. involving pressure, temperature and phase compositions on the three axes. A typical plot is shown in fig. 7.2. Fig. 7.2 Three dimensional VLE phase diagram ...
Vapor-liquid Equilibrium Diagrams. Uses Simulink to compute Vapor-Liquid Equilibrium diagrams for ethanol-water binary mixture. To compute activity coefficients, program uses Van-Laar Model. This mixture presents a positive azeotrope. Requirements: · MATLAB Release: R12 · Simulink February 2, 2011 - Figure 3. T-χ diagram for a binary mixture. To calculate vapor-liquid equilibria for multicomponent nonideal systems, the phase equilibrium, Eqs. (6), are usually presented in the same form as Eq. (9). However, in contrast to the ideal case (Eq. (10)), for this case distribution coefficients ... Draw vapor-liquid equilibrium (VLE) and liquid-miscibility (LLE) phase diagrams, or calculate distillation processes. Over 300 organic compounds. Vapor-Liquid Equilibrium Diagram for Non-Ideal Mixtures. This simulation shows P-x-y and T-x-y diagrams for vapor-liquid equilibrium (VLE) of a benzene/ethanol mixture. This liquid mixture is non-ideal, and the system has an azeotrope (a condition where liquid and vapor have the same composition). The blue curve represents the liquid-phase ...
Psub>xyand Tsub>xydiagrams give us information on vapor-liquid equilibrium. This is denoted by the enclosed yellow section on this graph. The yellow region constitutes the two phase (vapor and liquid) region. The red curve designates the bubble point curve, where the first formulation of a vapor occurs. The vapor-liquid equilibrium data is also plotted in a separate diagram. For example it will take about 75 kJ to change 1 kg of air at 25oC and 1 atm to oC and 1 atm. It will take much more energy, about kJ, to change 1 kg of water at oC and 1 atm to water vapor (steam) at the same temperature and pressure. Vapor-Liquid Equilibrium Data. The VLE for non-ideal systems can ... of vapour. Samples of equilibrium phases are taken at low temperature to avoid loss of components. From the quantitative analysis of the samples of the two phases it is possible to know the composition of the phases in equilibrium in every boiling temperature of the mixture and a TXY diagram of the binary ... Your password has been changed · Can't sign in? Forgot your password
Therefore, according to phase rule, for a vapour-liquid system with K components we get, N = K + 2 - ∅= K +2 - 2 = K. In other words, it means the numbers of parameters which we can change without disturbing the equilibrium state of the that system is equal to the number of components K. So, for a binary system N = 2, hence we can ...
Vapor-liquid equilibrium, abbreviated as VLE by some, is a condition where a liquid and its vapor (gas phase) are in equilibrium with each other, a condition or state where the rate of evaporation (liquid changing to vapor) equals the rate of condensation (vapor changing to liquid) on a molecular level such that there is no net (overall) vapor-liquid interconversion.
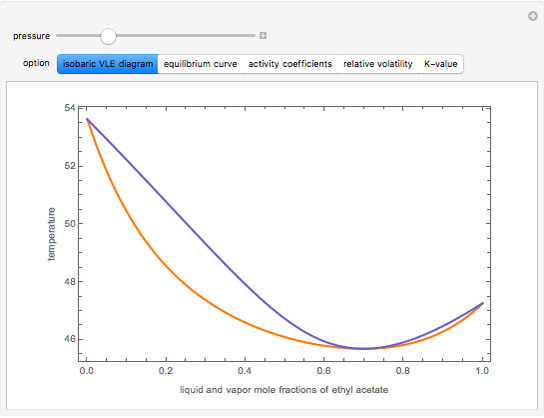
This Demonstration leads you through the construction of a temperature-composition ( - -) diagram step by step for vapor-liquid equilibrium (VLE) of an -hexane/ -octane ideal mixture (Raoult's law). Click the "new problem" button to construct a diagram at a different pressure. After completing each step, check the "solution" box to see the ...
August 11, 2020 - Because the molecules of a liquid are in constant motion and possess a wide range of kinetic energies, at any moment some fraction of them has enough energy to escape from the surface of the liquid …
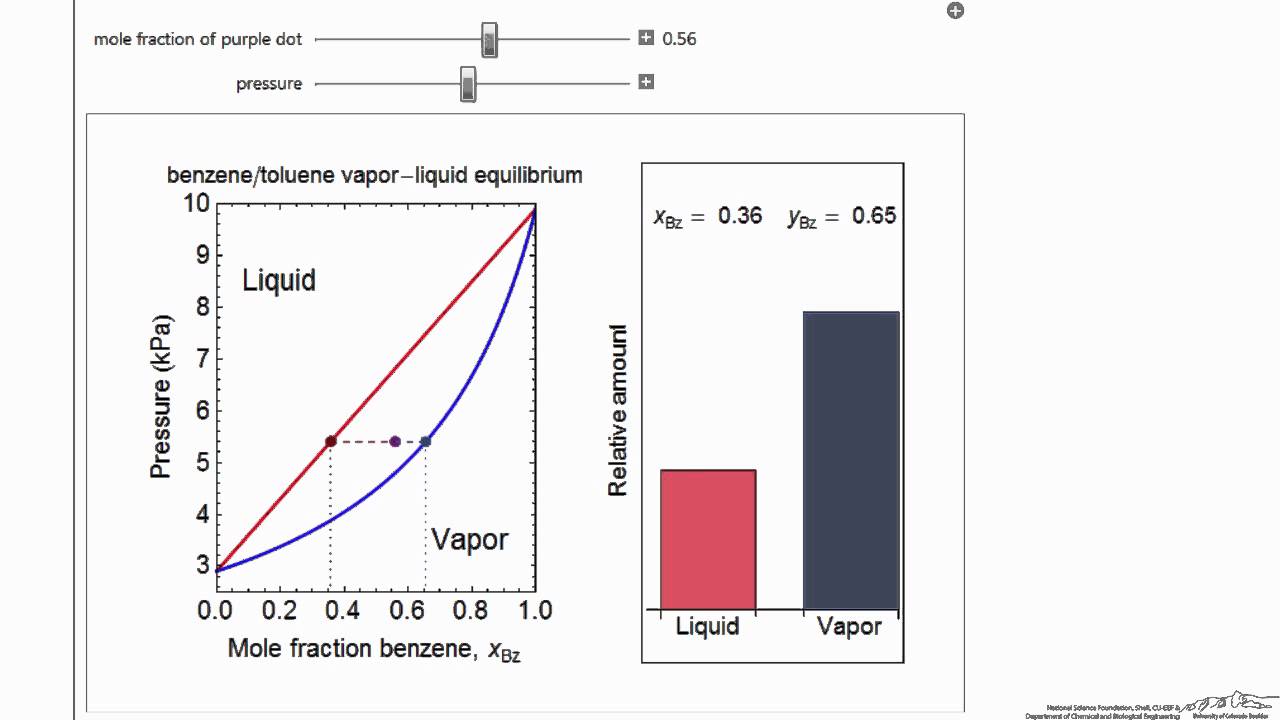
« Thermodynamics 2 simulations Description Instructional video Description The vapor-liquid equilibrium (VLE) behavior of a benzene (C 6H6)/toluene (C7H8) mixture is demonstrated in P-x-y and T-x-y…
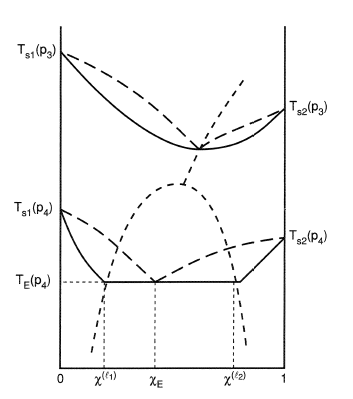
A schematic three-dimensional diagram illustrating these surfaces for VLE is shown in Fig. 10.1. This figure shows schematically the P-T-composition surfaces which contain the equilibrium states of saturated vapor and saturated liquid for a binary system.
Export articles to Mendeley · Get article recommendations from ACS based on references in your Mendeley library
Uses an interactive simulation to explain how to read a T-x-y diagram for a binary mixture that obeys Raoult's law.
3.3 Phase Diagram for Water Vapor: Clausius-Clapeyron Equation. The Clausius-Clapeyron Equation. We can derive the equation for e s using two concepts you may have heard of and will learn about later: entropy and Gibbs free energy, which we will not go into here.Instead, we will quote the result, which is called the Clausius-Clapeyron Equation,
Multiple-Component Phase Equilibrium: Phase Diagrams[edit | edit source] In general, chemical engineers are not dealing with single components; instead they deal with equilibrium of mixtures. When a mixture begins to boil, the vapor does not, in general, have the same composition as the liquid.
The preceding equilibrium equations are typically applied for each phase (liquid or vapor) individually, but the result can be plotted in a single diagram. In a binary boiling-point diagram, temperature (T ) (or sometimes pressure) is graphed vs. x1. At any given temperature (or pressure) where ...
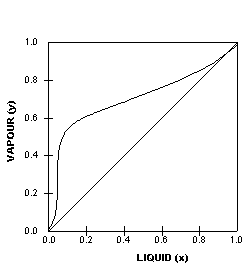
Thus the sizes, particularly the height, of distillation columns are determined by the vapour liquid equilibrium (VLE) data for the mixtures. Vapour-Liquid-Equilibrium (VLE) Curves. Constant pressure VLE data is obtained from boiling point diagrams. VLE data of binary mixtures is often presented as a plot, as shown in the figure on the right.
The phase diagram depends upon the Gibbs energies of vaporization of the pure components, Δ gv (Zn)o = ( μZnv − μZno (l)) and Δ gv (Mg)o = ( μMgv − μMgo (l)) as shown in Fig. 5.3. Sign in to download full-size image Fig. 5.3. Pressure-composition phase diagram of the Zn-Mg system at 977°C calculated for ideal vapor and liquid solutions.
Single Equilibrium Stages ()(1) [Ch.4][Ch. 4] •Phase Separation •Degree of Freedom Analysis-Gibbs phase rule F C P 2-General analysis •Binary Vapor-Liquid Systems-Examples of binary system-Phase equilibrium diagram-q-line-Phase diagram for constant relative volatility •AzeotropicSystems
on q or the liquid fraction of the feed. In the distillation literature, q is usually referred to as the thermal state of the feed. Note that q can be less than zero (superheated vapor) or greater than unity (subcooled liquid). The slope of the q-line is q/(q – 1). The construction of the two operating lines and the
Liquid-Vapor Equilibrium of a Binary System. Adapted by J. M. McCormick from an exercise used at the University of Kansas. Last Update: December 19, 2012. Introduction. The understanding of the equilibrium between the liquid and vapor phases in a multi-component system is important industries ranging from brewing to petroleum refining.
June 5, 2014 - The vaporliquid equilibrium VLE behavior of an hexaneoctane mixture is demonstrated in and diagrams The blue line represents the liquidphase boundary bubble point and the green line represents the vaporphase boundary dew point Click and drag the black dot on either diagram and the bar chart ...
The vapor liquid equilibrium data of water+MEA were measured in the pressure range from 50.0 to 70.0 kPa and temperature range from 355.2 to 430.3 K. The measured data were correlated with the UNIQUAC and NRTL activity coefficient models. Key words: Vapor Liquid Equilibrium, Monoethanolamine, Water, Activity Coefficient, Thermodynamic Consistency
Vapour-liquid equilibrium for a mixture of liquids is different from that of pure liquid. In a 2-component (binary) mixture, an equilibrium condition can exist between the vapour and the liquid phase. The Figure below showed a typical equilirbium condition attained under a certain pressure ...
Vapour liquid equilibrium 1 1. Vapour Liquid Equilibrium Definition: The vapor-liquid equilibrium (VLE) describes the distribution of a chemical species between the gas phase and a liquid phase. Explanation: The concentration of a vapor in contactwith its liquid, especially at equilibrium, is often expressed in terms of vapor pressure, which will be a partial pressure (a part of the total ...
The ability to accurately correlate vapor-liquid equilibria of pure substances is important, because systematic errors observed in such calculations will usually propagate into mixture calculations. Fig. 3 shows the vapor-liquid equilibrium of methane, propylene, diethylether and toluene in a T-ρ-diagram. The densities of the coexisting phases ...
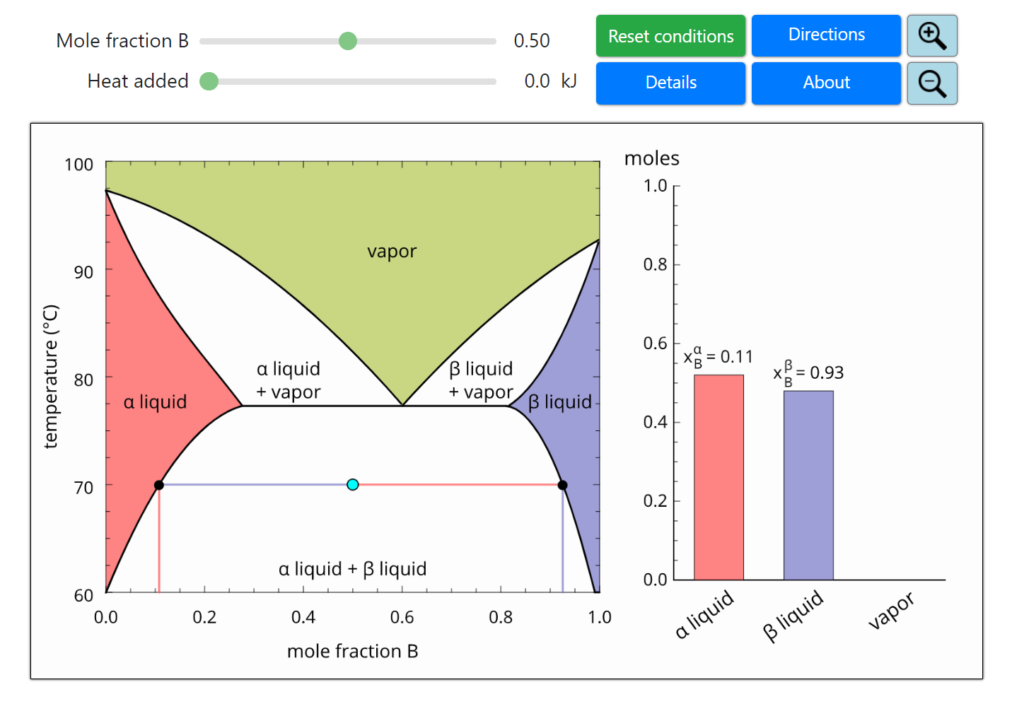
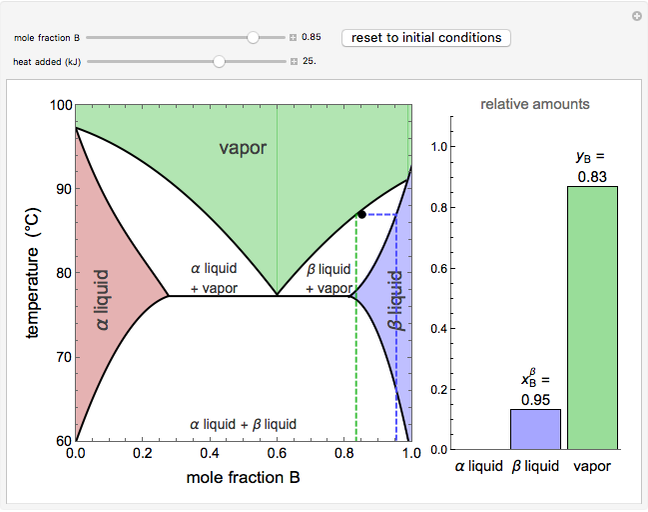
Vapor-Liquid-Liquid-Equilibrium Description This is a simulation of phase equilibrium for one mole of a binary system (A, B) that forms two partially-miscible liquids (α, β). The T-x-y diagram shows vapor-liquid equilibrium (VLE), liquid-liquid equilibrium (LLE), and vapor-liquid-liquid equilibrium (VLLE).
equilibrium. The recondensed vapor from the condensor drops back into the Claisen head, and some of it collects above the stockcock at c in the picture at right. We use a pipet filler to draw a small quantity of liquid (~1 mL) into the liquid sampler e, and collect the sample from the stopcock f. We will use a thermocouple
Vapour-liquid equilibria of the CH4 + CO2 + H2S ternary system with two different global compositions: experiments and modelling Pascal Theveneau1, Xiaochun Xu2, Olivier Baudouin3, Jean-Noël Jaubert2, Paola Ceragioli4, Christophe Coquelet1* 1 MINES ParisTech, PSL Université, CTP- Centre Thermodynamique des Procédés, 35 rue Saint Honoré, 77300 Fontainebleau France 2 Université de Lorraine ...
Vapor-liquid equilibrium data for Ethanol-Water* n-Buianol-Wator# Ethyl Aeeiate-Water# and n-Hexane-Ethanol at atmospheric pr 00 sura and £or n-Hoxana-Sthanol at 2f50 mnu, 59$ mm.* 1270 mm*# Ijbj mm** 2J10 mm.#
Vapor/Liquid Equilibrium • Previous chapters dealt with pure substances or with constant composition mixtures (air). • But in chemical reactions and number of industrially mass-transfer operations the . composition changes are the desired outcome. (Process such as distillation, absorption and extraction bring phases of different
A. Purpose - The objective of this experiment is to construct a liquid and vapor phase diagram of a binary or two-component system containing water and propanol. In order to determine the phase diagram, the temperature of the system must be measured during the experiment and the specific mole fractions must be obtained.
Even amid signs of an economic bounce, it's still wise for entrepreneurs not to tie up their capital. Signing out of account, Standby... Even amid signs of an economic bounce, it's still wise for entrepreneurs not to tie up their capital. H...
Vapor Liquid Equilibrium diagrams of binary systems (Continued) Figure M-55 Methanol - Benzene Figure M-81 Two propanol - Water Figure M-56 Methanol - Ethyl acetate Figure M-82 Water - 1 Butanol Figure M-57 Methanol - Isopropyl alcohol Figure M-83 Water - Acetic acid Figure M-58 Methanol - Water Figure M-84 Water - Formic acid ...
A liquid mixture is partially vaporized and the vapor is allowed to come to equilibrium with the liquid. The process flow diagram is shown in Fig. 3.1. The vapor and liquid phases are then separated. Fig. 3.1 Flash distillation. Making a component i balance, FxiF = Vyi + Lxi = Vyi + (F - V)xi (3-3) Defining f = V/F, eq. (7.3) becomes
The basis of distillation is phase equilibrium—specifically, vapor-liquid equilibrium (VLE) and in some cases vapor-liquid-liquid equilibrium (VLLE). Distillation can only ... represents the vapor composition. The second diagram is a plot of x versus y. These types of diagrams are generated at a constant pressure. Because the pressure in a
January 23, 2020 - Vapor–liquid equilibrium (VLE) data and modelling for LiBr+H2O and LiBr+CaCl2+H2O are reported in this paper. This work focuses on the experimental determination of boiling point for LiBr+H2O and LiBr+CaCl2+H2O solutions with vapor pressure between 6 and 101.3 kPa, the total molality of salt ...
Liquidation refers to the selling of assets in return for cash. Individuals, partnerships or corporations can liquidate assets. Here's how liquidation works. Liquidation refers to the selling of assets in return for cash. The term liquidati...
The yellow region constitutes the two phase (vapor and liquid) region. The red curve designates the bubble point curve, where the first formulation of a vapor ...
A conceptual question investigating how the molar composition changes to a vapor-liquid equilibrium system as temperature is changed. Made by faculty at the ...
Vapor Liquid Phase Diagrams. Vapor-liquid equilibrium data may be obtained by experiment, by thermodynamic calculation, or in published sources. It is typically presented either in tabular form or as an equilbrium diagram. Diagrams may take several forms: boiling point diagrams Txy, Pxy diagrams ternary diagrams solubility diagrams
Two types of vapor–liquid equilibrium diagrams are widely used to represent data for two-component (binary) systems. The first is a “temperature versus x and y” diagram (Txy). The x term represents the liquid composition, usually expressed in terms of mole fraction. The y term represents the vapor composition. The second diagram is a plot of
Calculation of vapor-liquid equilibrium (VLE) and drawing of phase diagrams. Name of substance. CAS-nr. Formula. Type of substance. acetone. 67-64-1. C 3 H 6 O. ketone.
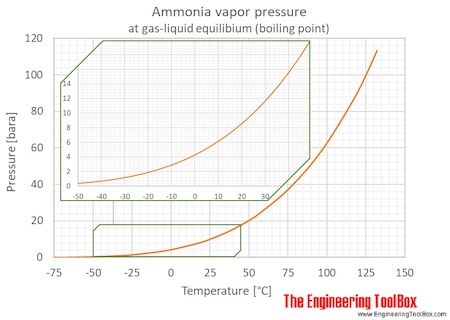

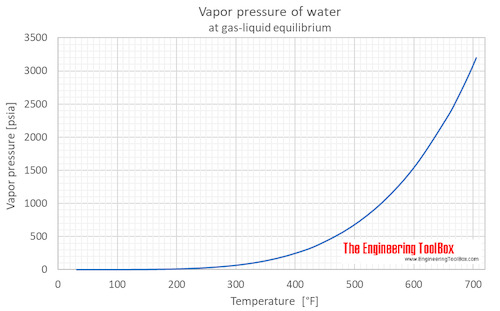
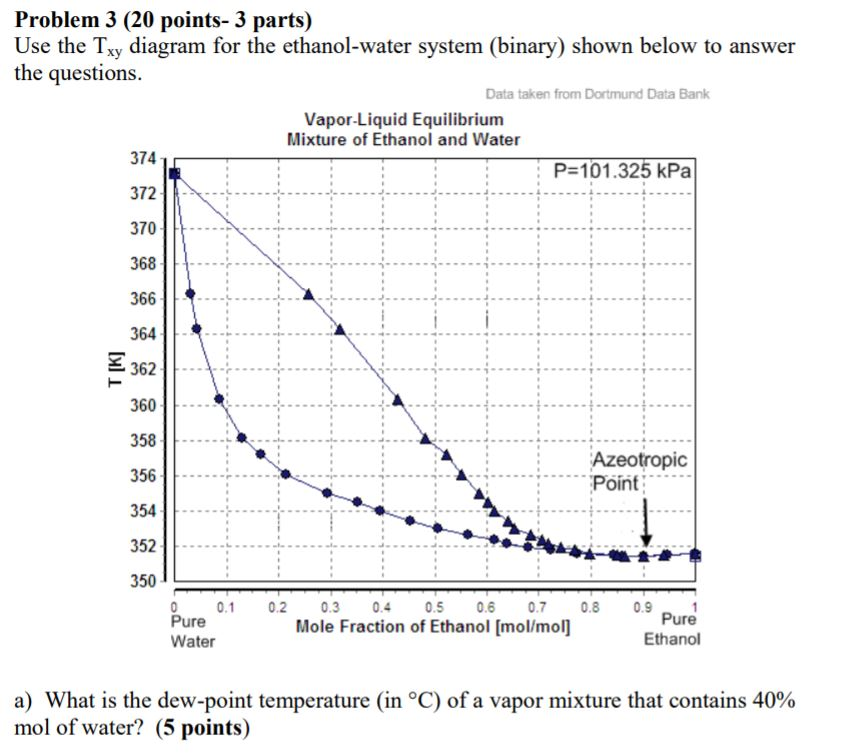
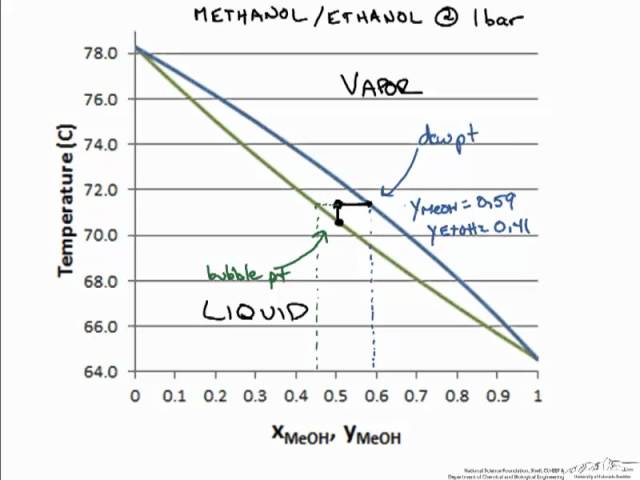










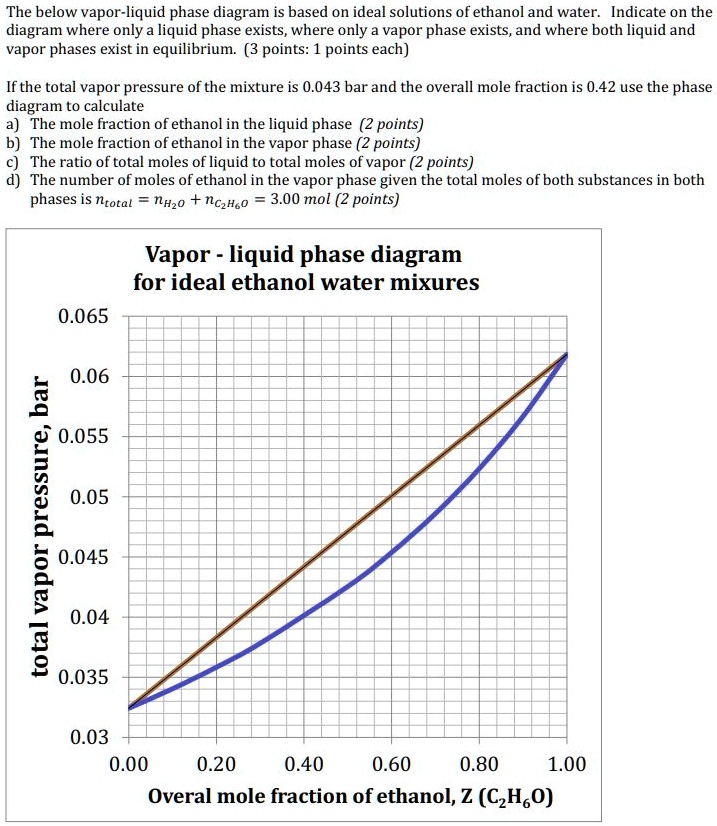
0 Response to "38 vapour liquid equilibrium diagram"
Post a Comment