38 two step reaction diagram
Answered: Draw a reaction energy diagram for a… | bartleby Science Chemistry Q&A Library Draw a reaction energy diagram for a two-step exothermic reaction whose second step is faster than the first step. Label the intermediate, transition states, ΔH, and activation energies. Welcome to CK-12 Foundation | CK-12 Foundation The slow step (reaction 2) is the rate-determining step. Whatever the reaction rate is for reaction 2, the overall rate will be exactly the same. Lesson Summary A multi-step reaction is a combination of two or more elementary steps. An elementary step is a single, simple step involving one or two particles.
Qualitative Energy Diagram - Diagram The potential energy diagram for a hypothetical one-step bimolecular reaction and for a two-step reaction are shown in Fig. E k kinetic energy E g. Draw an energy pie chart for each scenario A and B. Data Visualization With R. Qualitative Energy Flow Diagrams Practice Draw Energy Flow Diagrams for.
Two step reaction diagram
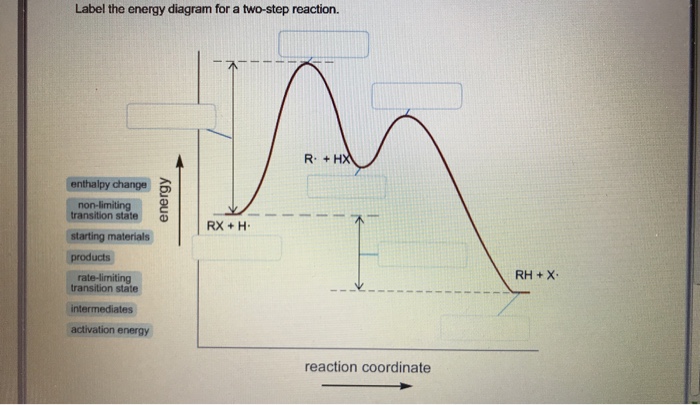
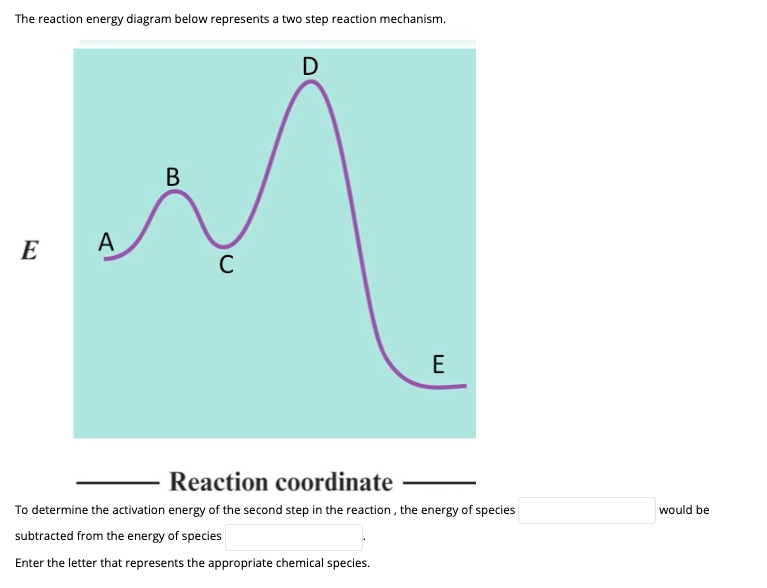
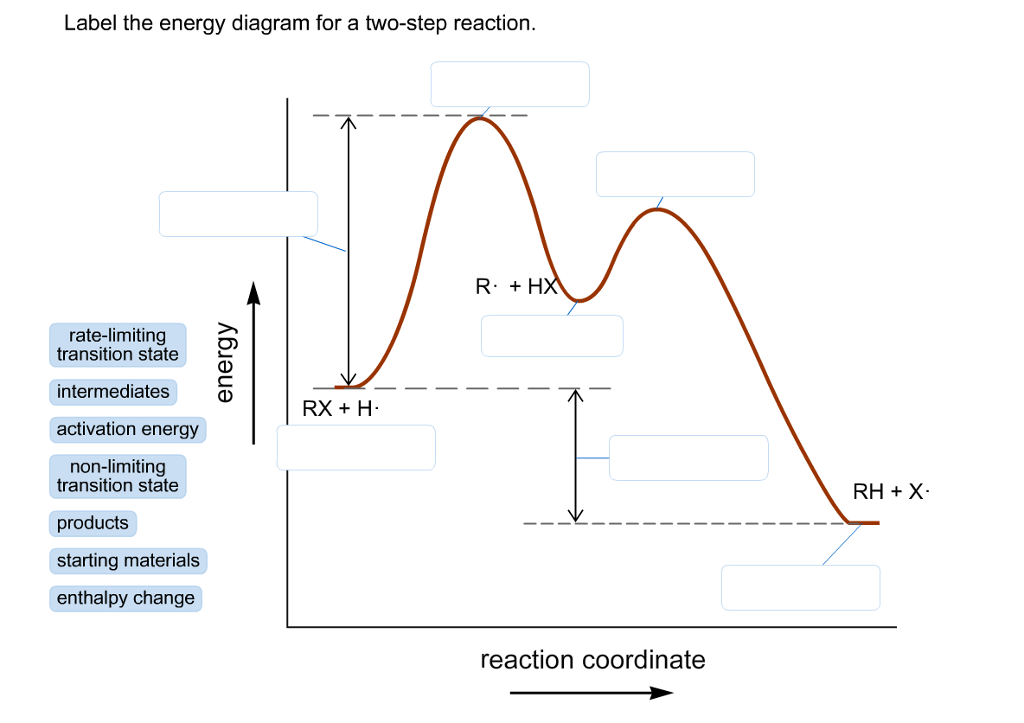
Solved Label the energy diagram for a two-step reaction ... See the answer See the answer done loading. Show transcribed image text. Expert Answer. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. 100% (42 ratings) Transcribed image text: Label the energy diagram for a two-step reaction. PDF Reactions of Alkenes and Alkynes - Bloomsburg Area School ... In a reaction that occurs in two or more steps, each step has its own transition state and activation energy. Shown in Figure 5.2 is an energy diagram for the conversion of reac-tants to products in two steps. A reaction intermediate corresponds to an energy minimum 2 step reaction coordinate diagram - YouTube goes through the SN1 reaction between 2-bromo-2-methylpropane and chloride ion to form 2-chloro-2-methylpropane and bromide ion. Details of reaction coordina...
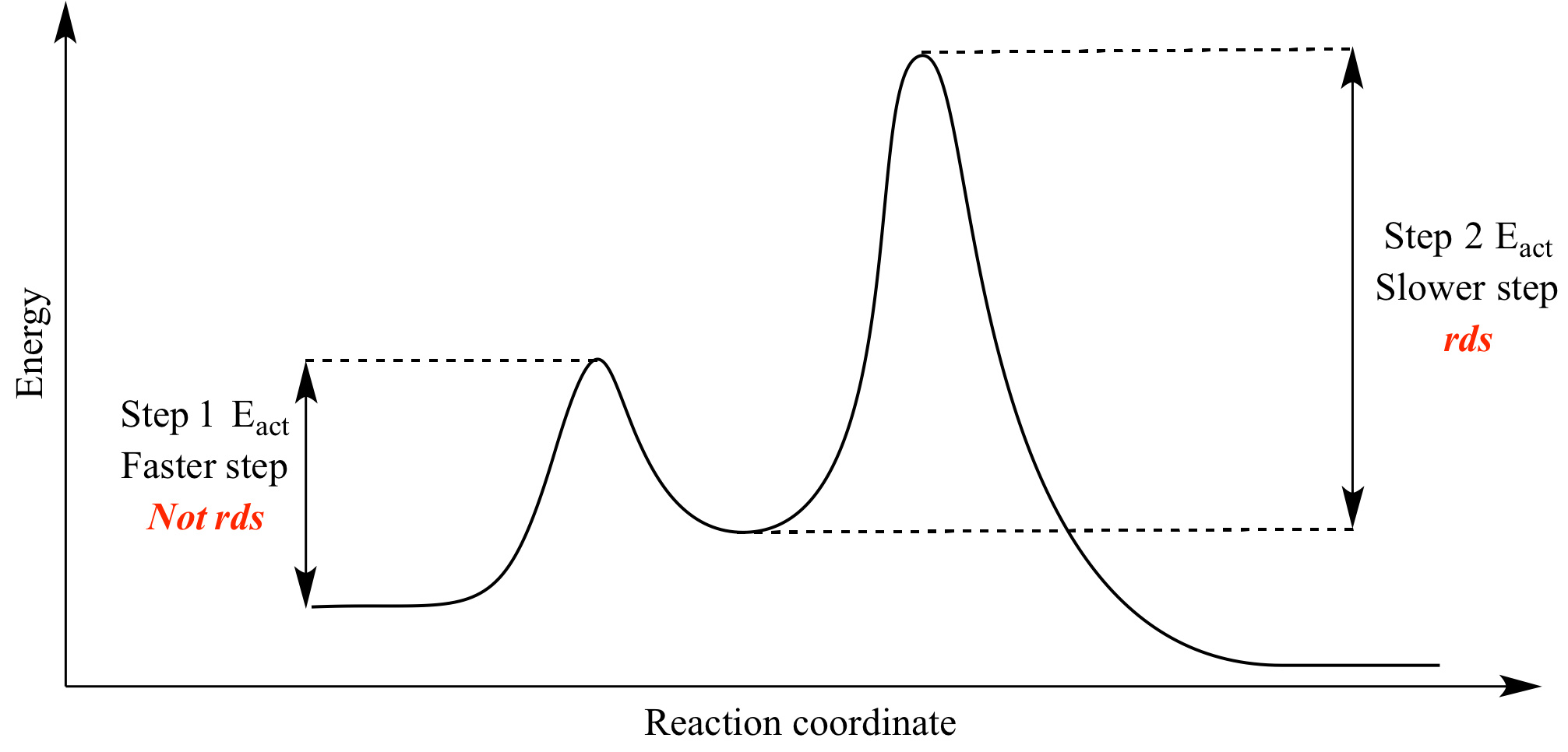
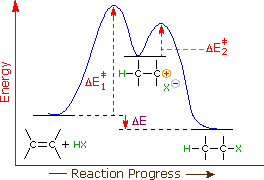
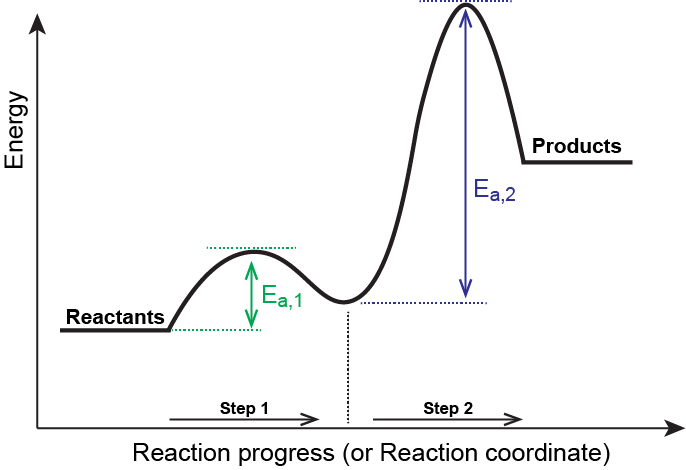
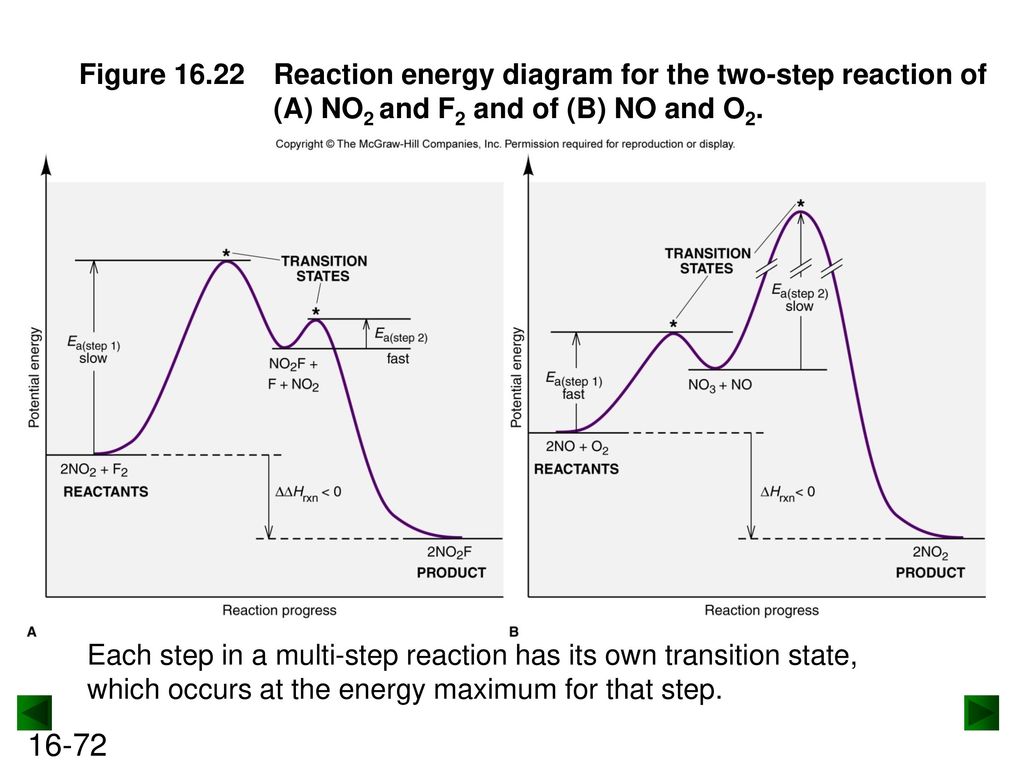
Two step reaction diagram. PDF AP CHEMISTRY 2013 SCORING GUIDELINES - College Board Step 1: C2H4(g) + HCl(g) C2H5+(g) + Cl (g) rate-determining step Step 2: C2H5 +(g) + Cl (g) C 2H5Cl(g) fast step (c) Write the rate law for the reaction that is consistent with the reaction mechanism above. rate = k[C2H4][HCl] 1 point is earned for the correct rate law. (d) Identify an intermediate in the reaction mechanism above. C2H5 +(g) or ... CH 368: Unit 2 - University of Texas at Austin As simple illustrations of stepwise mechanisms, we will consider two distinct scenarios for reactions which occur in two steps. First Step Rate-Determining. Mechanism: Reaction Path Diagram: The term "rate-determining step" or "rds" has a specific meaning. An rds is a step of a reaction the rate of which is equal to the rate of the ... Reaction Mechanisms and Multistep Reactions - Chemistry ... The three-step reaction depicted here involves two intermediate species I 1 and I 2, and three activated complexes numbered X 1-3. Although the step I 2 → products has the smallest individual activation energy E a 3 , the energy of X 3 with respect to the reactants determines the activation energy of the overall reaction, denoted by the ... Reaction Coordinate Diagrams The reaction coordinate diagram for the ozone photolysis reaction is a little different from those above because this is an endothermic reaction. Together, the products O 2 and atomic O, have a higher energy than the reactant O 3 and energy must be added to the system for this reaction.
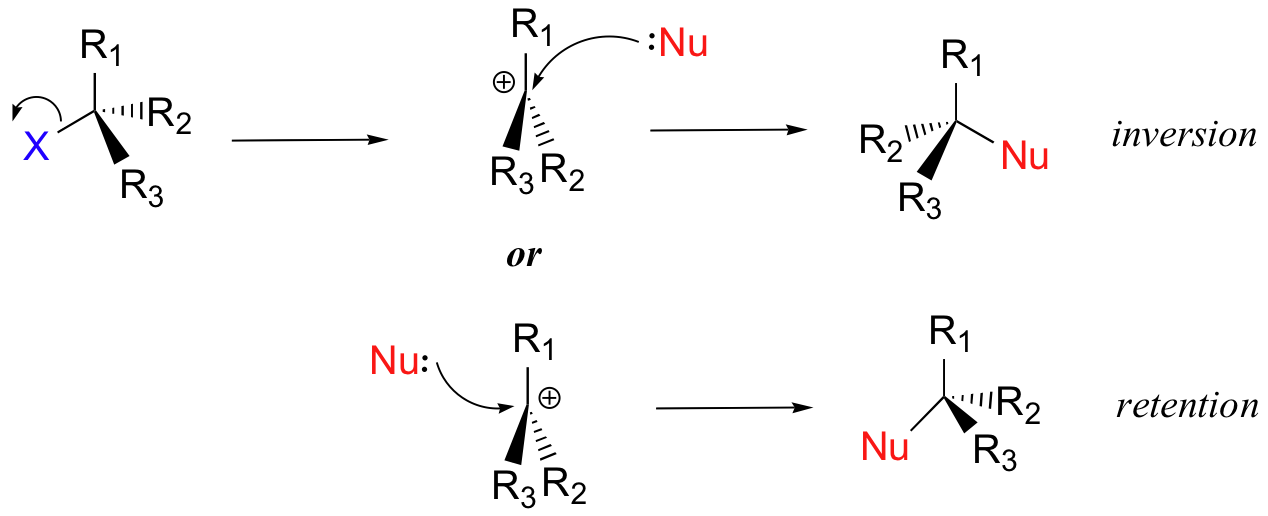
6.15: Energy Diagram for a Two-Step Reaction Mechanism ... 6.15: Energy Diagram for a Two-Step Reaction Mechanism Last updated; Save as PDF Page ID 93060; No headers. A second model for a nucleophilic substitution reaction is called the 'dissociative', or 'S N 1' mechanism: in this picture, the C-X bond breaks first, before the nucleophile approaches: This results in the formation of a carbocation: because the central carbon has only three bonds, it ... Energy Diagram for a Two-Step Reaction Mechanism by Ashima ... Energy Diagram for a Two-Step Reaction Mechanism Complete Energy Diagram for Two-Step Reaction A Two-Step Reaction Mechanism The transition states are located at energy maxima. The reactive intermediate B+ is located at an energy minimum. Each step has its own delta H and Lesson Worksheet:Reaction Profiles | Nagwa The reaction profile diagram for a two-step chemical reaction is shown below. In step 1, compound a reacts to form compound b, and in step 2, compound b reacts to form compound c. Which step has the highest activation energy? A Step 1 and step 2 have equal activation energies. B Step 2 C Step 1 Which step is an exothermic reaction? Draw an energy diagram for a two-step exergonic reaction ... in this question. We're going to draw the energy during um for two step reaction where the second step of the reaction is faster than the first step of the reaction. It's no. Let's recall the definition of Gibbs free energy change and that is the difference between the free energy of the products. My enough see free energy of the reactant. It's so yeah, the gifts rich free energy change is ...
12.7 Catalysis - Chemistry - opentextbc.ca Using Reaction Diagrams to Compare Catalyzed Reactions The two reaction diagrams here represent the same reaction: one without a catalyst and one with a catalyst. Identify which diagram suggests the presence of a catalyst, and determine the activation energy for the catalyzed reaction: Solution Identifying Steps in a Reaction Profile Diagram for a Two ... The reaction-profile diagram for a two-step chemical reaction is shown below. In step 1, compound a reacts to form compound b, and in step two, compound b reacts to form compound c. Which step has the highest activation energy? Which step is an exothermic reaction? Exothermic Energy Diagram: Activation Energy, Transition ... In this video, I go over how to properly label and explain a reaction mechanism diagram which is also referred to as an energy diagram or energy graph. I'll ... Energy Diagrams: Describing Chemical Reactions Draw an energy diagram for a two-step reaction that is exothermic overall, and consists of a fast but endothermic first step, and a slow but exothermic second step. Indicate DG rxn, as well as DG 1 * and DG 2 * for the first and second activation energies, respectively. Label the positions corresponding to the transition states with an asterisk.
PDF 10-17-19 Reactions of Alkenes - Michigan State University Energy Diagram for a Two-Step Reaction Involving Formation of an Intermediate Reactive intermediate corresponds to an energy minimum between two transition states Rate-determining step: Step that crosses the highest energy barrier: Slowest step in a multistep reaction
Draw an energy diagram for a two-step exergonic reaction whose Draw an energy diagram for a two-step exergonic reaction whose. Need more help! Draw an energy diagram for a two-step exergonic reaction whose second step is faster than its first step. Students also viewed these Organic Chemistry questions. Draw an energy diagram for a one-step reaction with Keq 1.
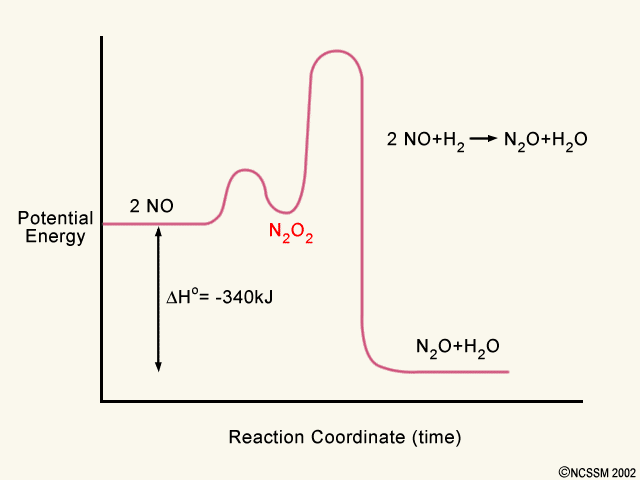
6. Reaction Coordinate Diagram - VIZISCIENCE® INTERACTIVE LABS The reaction involves two steps, step 1 is the slowest step and step 2 is the fastest step. Both steps are exothermic. Indicate on the diagram the overall enthalpy change of the reaction, the reaction for the transition states and intermediate states. The reaction is a reaction between hydrogen gas and brown vapor of iodine monochloride.
12.7 Catalysis - Chemistry 2e - OpenStax The catalyzed reaction is the one with lesser activation energy, in this case represented by diagram b. Check Your Learning Reaction diagrams for a chemical process with and without a catalyst are shown below. Both reactions involve a two-step mechanism with a rate-determining first step.
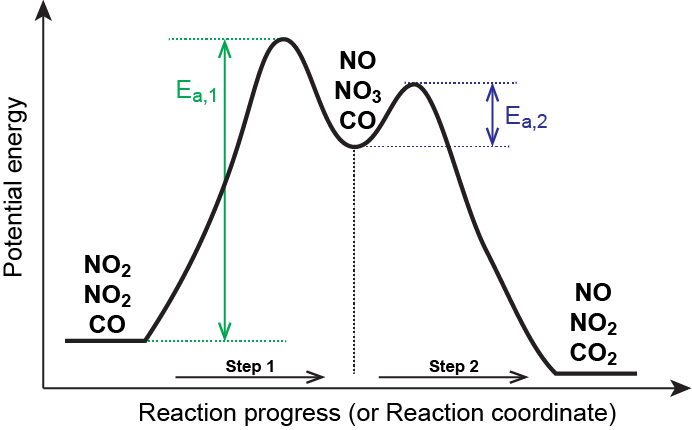
Multistep Reactions - Softschools.com The energy diagram of a two-step reaction is shown below. In the above reaction, a reactant goes through one elementary step with a lower activation energy (transition state 1) to form the intermediate. The intermediate then goes through a second step (transition state 2) with the highest energy barrier to form the product.
Energy Diagrams of Two Step Reactions - YouTube Watch Complete videos @ Organic Chemistry 1
Solved: Sketch an energy diagram for a two-step reaction ... Sketch an energy diagram for a two-step reaction in which both steps are exergonic and in which the second step has a higher-energy transition state than the first. Label the parts of the diagram corresponding to reactant, product, intermediate, overall Δ G‡, and overall Δ G °. Step-by-step solution 89% (9 ratings) for this solution Step 1 of 4
Draw a reaction-energy diagram for a two-step endothermic ... Solution for Draw a reaction-energy diagram for a two-step endothermic reaction with a rate-limiting second step. close. Start your trial now! First week only $4.99! arrow_forward. learn. write. tutor. study resourcesexpand_more. Study Resources. We've got the study and writing resources you need for your assignments. ...
Reaction Coordinate - Two Step Exothermic Reaction 002 ... Draw a reaction coordinate for the following catalyzed two-step exothermic reaction with the second step being the rate determining step:A -- B
2 step reaction coordinate diagram - YouTube goes through the SN1 reaction between 2-bromo-2-methylpropane and chloride ion to form 2-chloro-2-methylpropane and bromide ion. Details of reaction coordina...
PDF Reactions of Alkenes and Alkynes - Bloomsburg Area School ... In a reaction that occurs in two or more steps, each step has its own transition state and activation energy. Shown in Figure 5.2 is an energy diagram for the conversion of reac-tants to products in two steps. A reaction intermediate corresponds to an energy minimum
Solved Label the energy diagram for a two-step reaction ... See the answer See the answer done loading. Show transcribed image text. Expert Answer. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. 100% (42 ratings) Transcribed image text: Label the energy diagram for a two-step reaction.

















0 Response to "38 two step reaction diagram"
Post a Comment