38 orbital diagram for aluminum
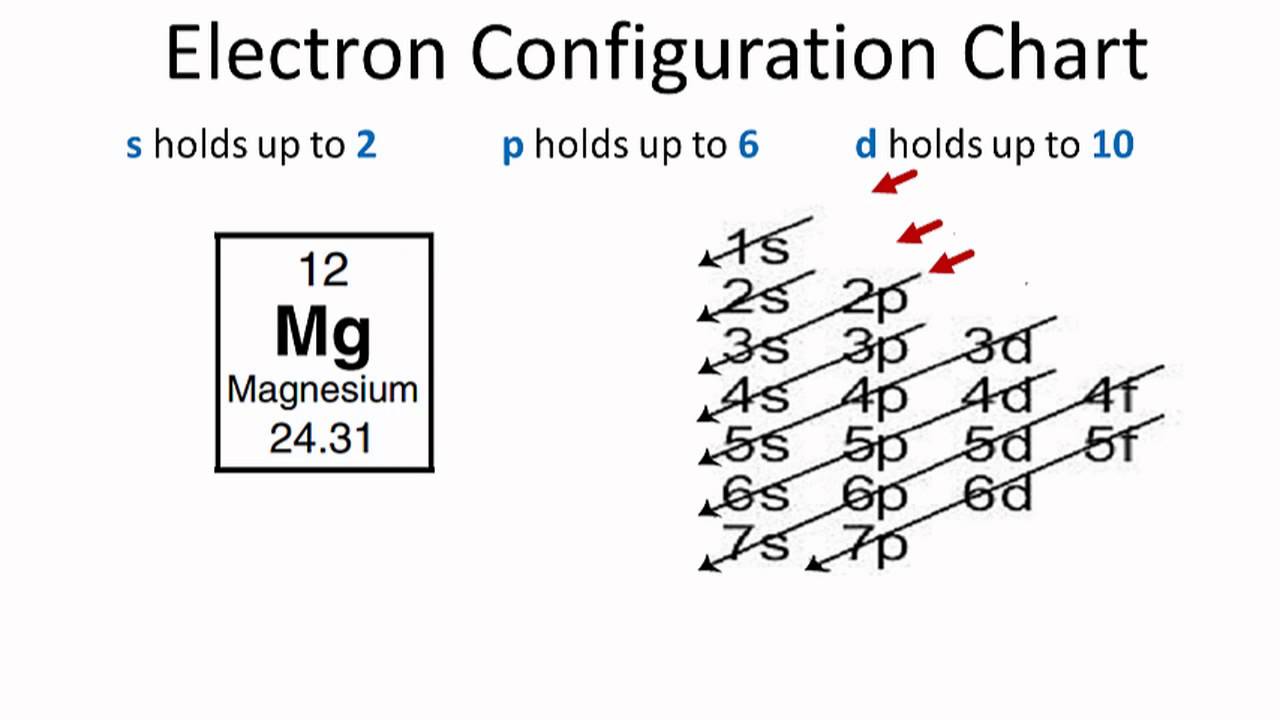
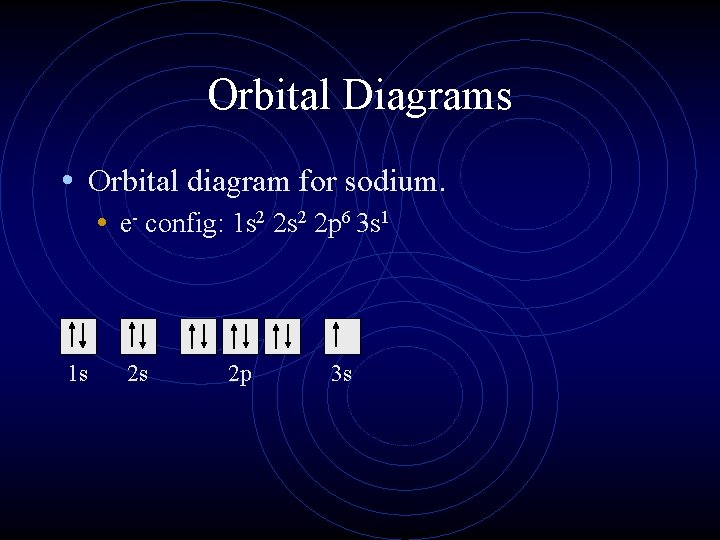
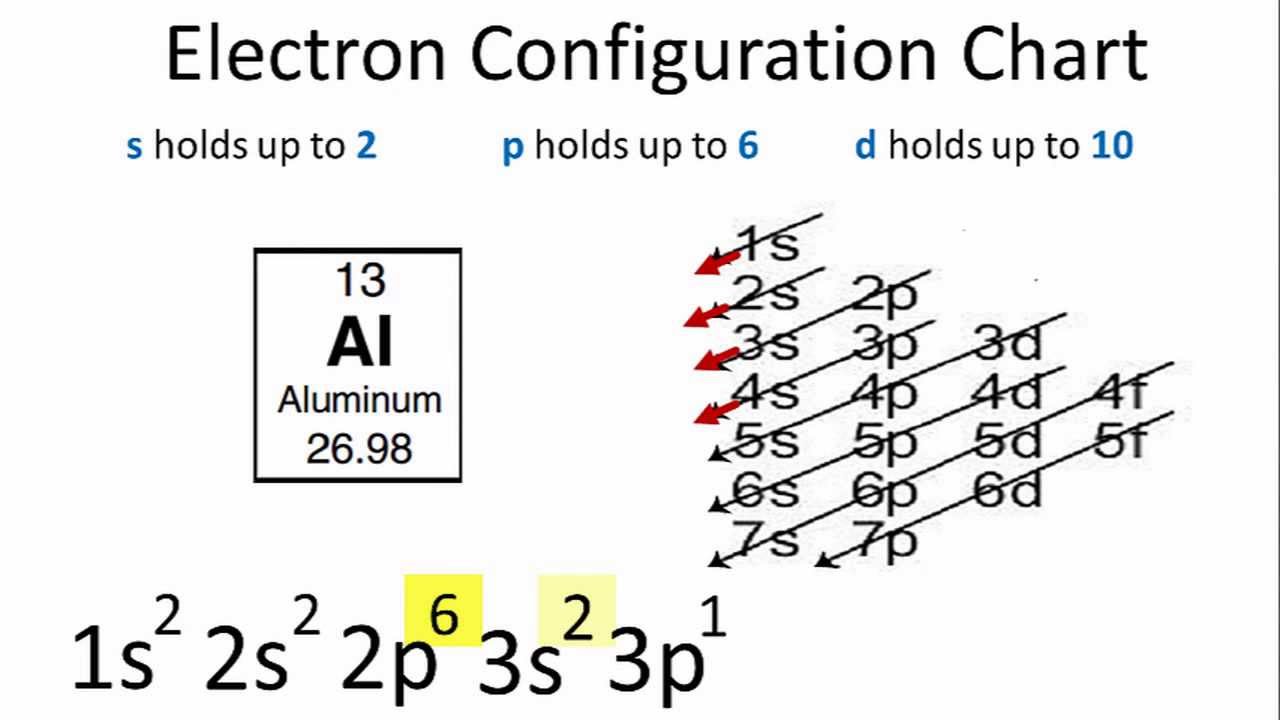
So based on what we know about the quantum numbers and using the chart above, you need 2 electrons to fill an s orbital, 6 electrons to fill a p orbital, 10 electrons to fill a d orbital and 14 electrons to fill the f orbital. BUT what we haven't discussed is how these orbitals get filled...the order of fill. Order of Fill In writing the electron configuration for Aluminium the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for aluminium go in the 2s orbital. The nex six electrons will go in the 2p orbital. The p orbital can hold up to six electrons.
To write the orbital diagram for the Aluminum atom (Al) first we need to write the electron configuration for just Al. To do that we need to find the number ...

Orbital diagram for aluminum
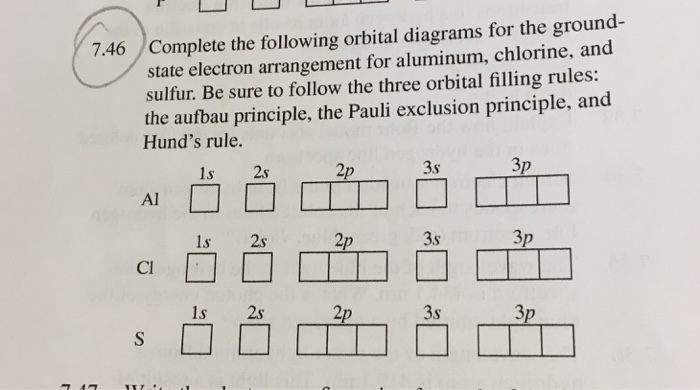
Write the orbital diagram of aluminum. 4. Write the complete electron configuration and the noble-gas notation for aluminum. 5. Write the noble-gas notation for iodine. 6. Identify each atom. a. 1s22s22pl b. [Ar]4sl carbon sulfur 7. Write electron-dot structures for the following atoms. A Bohr diagram is a simplified visual representation of an atom that was developed by Danish physicist Niels Bohr in The diagram depicts the atom as a positively charged nucleus surrounded by electrons that travel in circular orbits about the nucleus in discrete energy levels. Jan 18, · Aluminum, Al Bohr Diagram. An orbital diagram is similar to electron configuration, except that instead of indicating the atoms by total numbers, each orbital is shown with up and down arrows to represe nt the electrons in each orbital. Refer to the related link to see an illustration of an orbital diagram for aluminum.
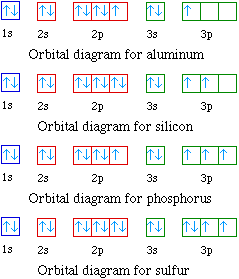
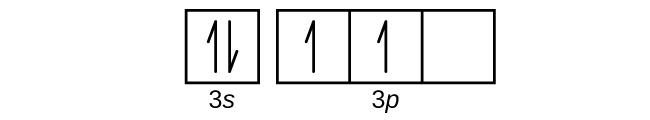
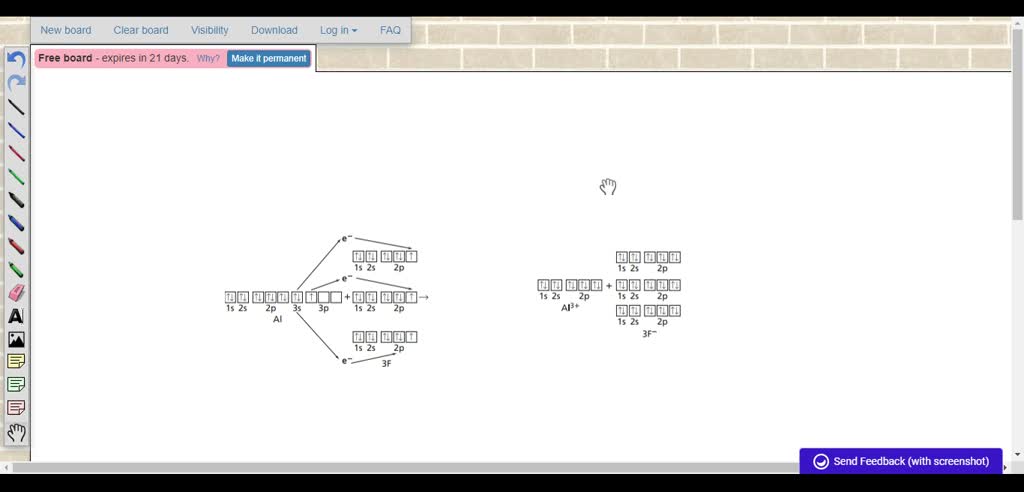
Orbital diagram for aluminum. The orbital diagram of aluminum helps show the specific "address" of each electron. Each arrow in the diagram represents a single electron (arrow pointing up if positive spin, down if negative spin). The numbers above the squares of the diagram represent the energy level, and the letters represent sub levels. Each box represents an orbital, also. The electron configuration for aluminum is: 1s^2 2s^2 2p^6 3s^2 3p^1 To figure out the electron configuration of any element you will use the diagonal diagram (seen in the right side of video below) and use the Aufbau principle . This video discusses how to write electron configurations for H, Li, C and Sc. Hope this helps! To determine the electron configuration and draw an orbital diagram of aluminum, we follow 3 rules: Aufbau principle: This states we fill starting from lower energy levels and progress to the next ... Question. : Fill in the orbital energy diagram for aluminum 3p 3s 2p 1s 1A H 2A Li Be 8A 3A 4A 5A 6A 7A He BCNOFNe Mn Fe Co Ni Cu zn Ga Ge As Se Br Kr Cs Ba La Hf Ta W Re Os Ir Pt Au Hg T Pb Bi Po At Rn Write the complete electron configuration for the vanadium atom Using NOBLE GAS notation write the electron configuration for the nickel atom.
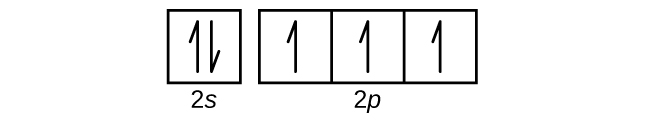
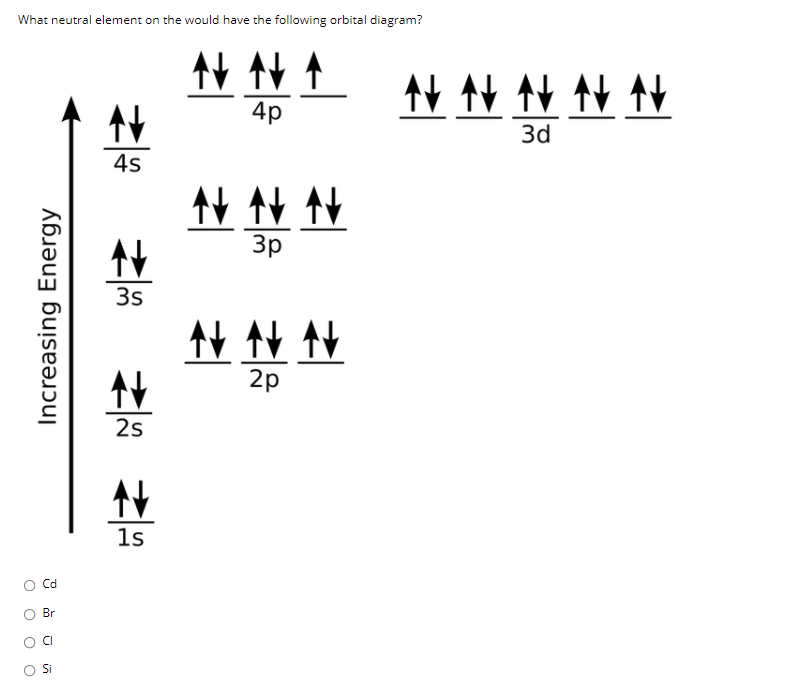
Oct 19, 2009 · An orbital diagram is similar to electron configuration, except that instead of indicating the atoms by total numbers, each orbital is shown with up and down arrows to represent the electrons in ... - The SHAPE of an orbital is defined by the SUBSHELL it is in ... 159 ENERGY DIAGRAM - We can map out electrons around an atom using an energy diagram: E N E R G Y 1s 2s 2p 3s 3p 3d 4s 4p 4d 5s 5p ... Aluminum: Z = 13 Aluminum has THREE valence electrons! (All electrons in the outer shell are valence Orbital Filling Diagrams •Each box represents an orbital which can hold a max of 2 e- •Aufbau principal -each electron occupies the lowest energy orbital available; German for "build up" •Electrons are notated with an arrow -Up arrow goes first then, down arrow -Arrows represent the opposing spin of electrons 5.2 Quantum Theory & The Atom Answer: Aluminum (Al) has only atomic orbitals. As a 3rd row element, however, it has a complete Ne electron configuration. One way to denote this is to write it as the following: [Ne] 3s(2) 3p(1), The parentheses indicate the number of electrons in each orbital. The full electron shell (includ...
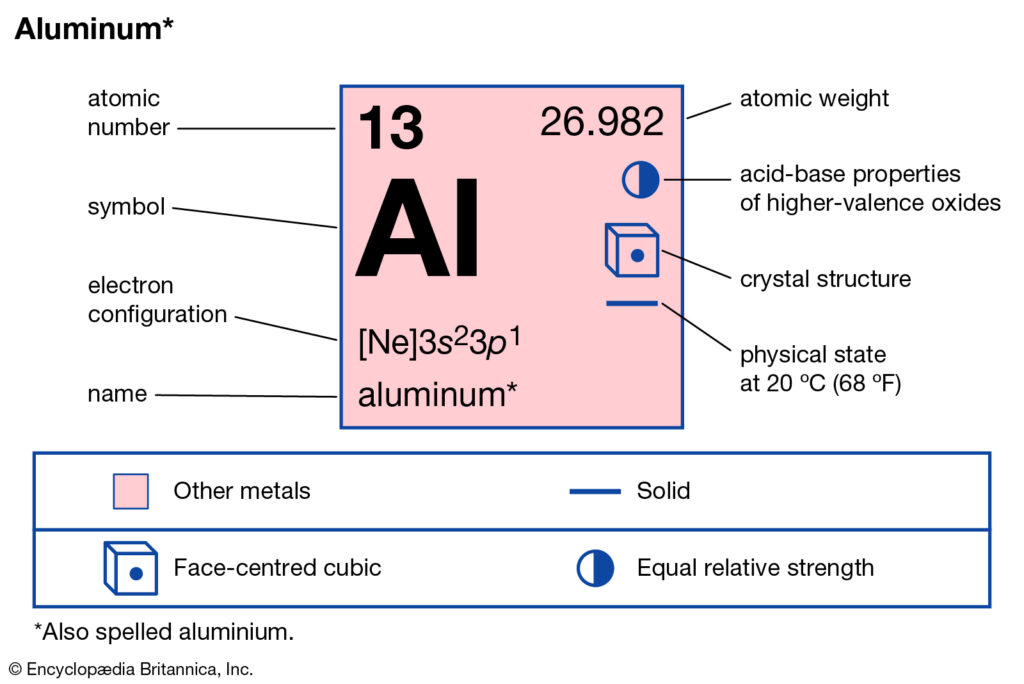
Aluminum (Al, US spelling). Diagram of the nuclear composition, electron configuration, chemical data, and valence orbitals of an atom of aluminum-27 (atomic number: 13), the most common isotope of this element. The nucleus consists of 13 protons (red) and 14 neutrons (orange). 13 electrons (white) occupy available electron shells (rings). Aluminum(Al) is the 13th element in the periodic table and its symbol is ‘Al’. The electron configuration of aluminum and the orbital diagram is the main topic of this article. Also, valency and valence electrons of aluminum, various reactions, and compound formation, bond formation have been discussed. Hopefully, after reading this article ... Please draw the orbital diagram for Aluminum. Based on your drawing, is aluminum attracted to a manget? Aslo, Draw the orbital diagram for Fe3+. Again, based on your orbital diagram for Fe3+, is this ion attracted to a magnet? Manganese (Mn) excited state electron configuration and orbital diagram. When a manganese atom is excited, then the manganese atom absorbs energy. As a result, an electron in the 4s orbital jumps to the 4p x sub-orbital. The p-orbital has three sub-orbitals. The sub-orbitals are p x, p y, and p z.
What is the orbital filling diagram for aluminum? The electron configuration of aluiminium is: [Ne]3s23p1. What is an orbital diagram? An orbital diagram is similar to electron configuration,...
Answer: Aluminum (Al) has only atomic orbitals. As a 3rd row element, however, it has a complete Ne electron configuration. One way to denote this is to write it as the following: [Ne] 3s(2) 3p(1), The parentheses indicate the number of electrons in each orbital. The full electron shell (includ...
The electron configuration of aluminum and the orbital diagram is the main topic of this article…. Boron (B) electron configuration with full orbital diagram Boron is the 5th element in the periodic table and the first element in group-13. The atomic number of boron is 5 and its symbol is 'B'. The standard atomic…
Orbital diagram of Carbon (C) 7: Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine (F) 10: Orbital diagram of Neon (Ne) 11: Orbital diagram of Sodium (Na) 12: Orbital diagram of Magnesium (Mg) 13: Orbital diagram of Aluminum (Al) 14: Orbital diagram of Silicon (Si) 15: Orbital diagram of Phosphorus ...
An orbital diagram is similar to electron configuration, except that instead of indicating the atoms by total numbers, each orbital is shown with up and down arrows to represe nt the electrons in each orbital. Refer to the related link to see an illustration of an orbital diagram for aluminum.
A Bohr diagram is a simplified visual representation of an atom that was developed by Danish physicist Niels Bohr in The diagram depicts the atom as a positively charged nucleus surrounded by electrons that travel in circular orbits about the nucleus in discrete energy levels. Jan 18, · Aluminum, Al Bohr Diagram.
Write the orbital diagram of aluminum. 4. Write the complete electron configuration and the noble-gas notation for aluminum. 5. Write the noble-gas notation for iodine. 6. Identify each atom. a. 1s22s22pl b. [Ar]4sl carbon sulfur 7. Write electron-dot structures for the following atoms.





















0 Response to "38 orbital diagram for aluminum"
Post a Comment