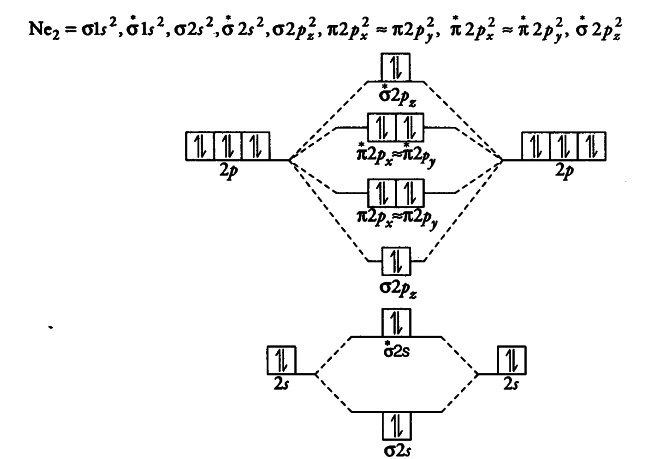
38 Molecular Orbital Diagram For Ne2
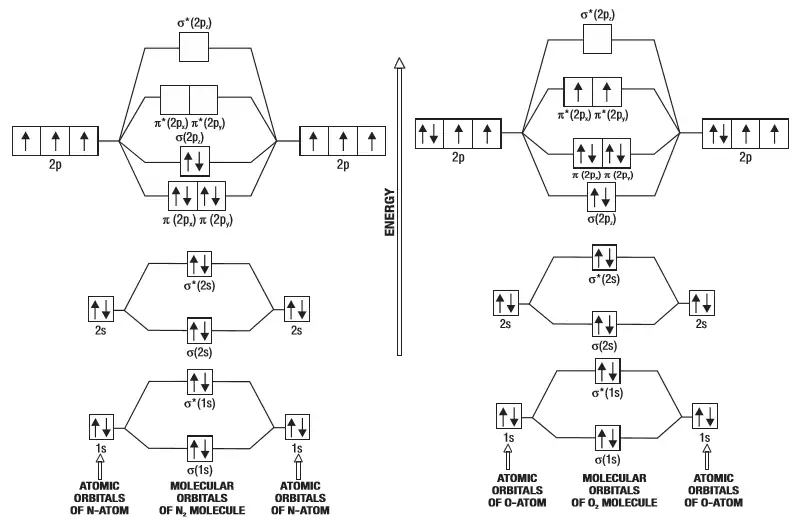
Use the molecular orbital energy level diagram to show that N2 Use the molecular orbital energy level diagram to show that N2 would be expected to have a triple bond, F2, a single bond and Ne2, no bond. · Solution.1 answer · Top answer: Formation of N2 molecule: Electronic Configuration, σ 1s^2<σ *1s^2<σ 2s^2<σ *2s^2<[pi 2px^2 = pi 2px^2]<<σ 2pz^2 Bond order = (Nb - Na)/2 = (10 - ... Asked for: "skewed" molecular orbital energy-level diagram, bonding... When we draw a molecular orbital diagram for a molecule, there are four key points to remember: The number of molecular orbitals produced is the same as the number of atomic orbitals used to We illustrate how to use these points by constructing a molecular orbital energy-level diagram for F2.
Molecular Orbital Theory (MOT), Chemistry Study... | eMedicalPrep The molecular orbital diagram representing this order of energy levels is shown in fig. No. 9 Molecular Orbital Diagram for CO. Analysis done by Bond Order. If value of bond order is positive, it indicates a stable molecule and if the value is negative or zero, it means that the molecule is unstable.

Molecular orbital diagram for ne2
PDF Microsoft PowerPoint - An introduction to Molecular Orbital Theory.ppt... - MO diagrams for Inorganic complexes. • It is a waste of both the lecturers and students time if the tutorial to ends up being a lecture covering questions. 5. An introduction to Molecular Orbital Theory. 1000 Solved Problems in Classical Physics - Kamal - Studylib Biblioteca en línea. Materiales de aprendizaje gratuitos. 1000 Solved Problems in Classical Physics Ahmad A. Kamal 1000 Solved Problems in Classical Physics An Exercise Book 123 Dr. Ahmad A. Kamal Silversprings Lane 425 75094 … Molecular Orbital Theory This molecular orbital model can be used to explain why He2 molecules don't exist. Combining a pair of helium atoms with 1s2 electron configurations would The molecular orbital diagram for an O2 molecule would therefore ignore the 1s electrons on both oxygen atoms and concentrate on the...
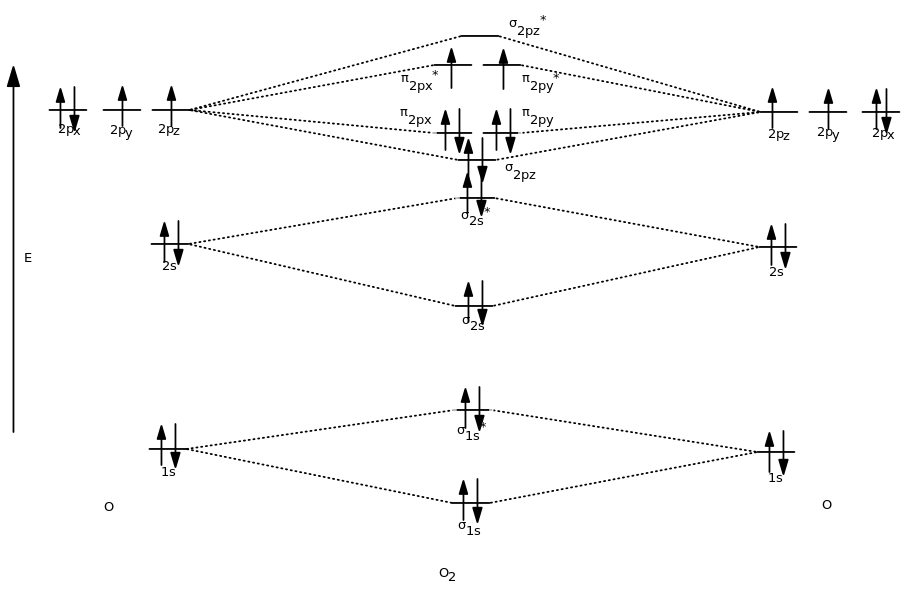
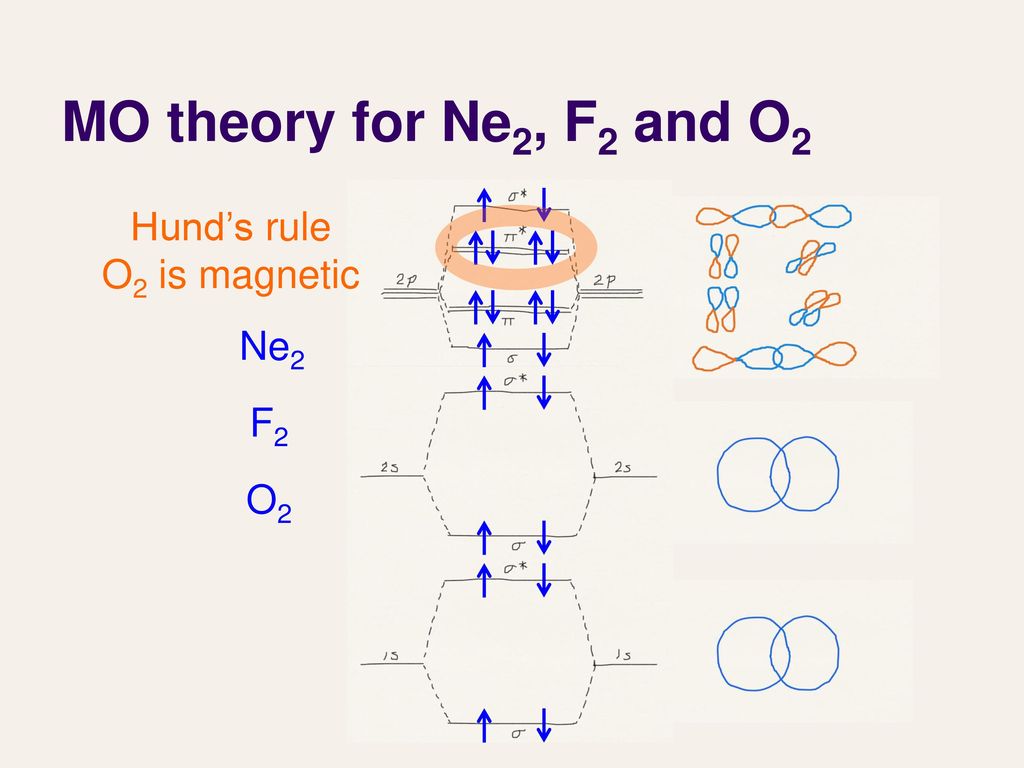
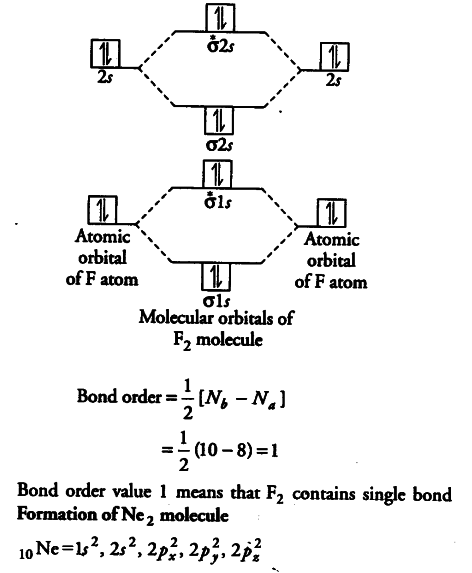
Molecular orbital diagram for ne2. Figure 11. The molecular orbital energy diagram predicts that... Figure 9. This is the molecular orbital diagram for the homonuclear diatomic Be2+, showing the molecular orbitals of the valence shell only. The MOs for the valence orbitals of the second period are shown in Figure 12. Looking at Ne2 molecular orbitals, we see that the order is consistent with... Molecular Orbital Theory (5.4) - Chemistry 110 Looking at Ne2 molecular orbitals, we see that the order is consistent with the generic diagram shown in the previous section. Figure 5.52 This shows the MO diagrams for each homonuclear diatomic molecule in the second period. The orbital energies decrease across the period as the effective... With the help of molecular orbital diagram show that Ne2 ... Jan 26, 2020 — With the help of molecular orbital diagram show that Ne2 cannot exist as stable molecule. (Atomic number of Ne = 10). chemical bonding ...1 answer · Top answer: Ne2 (20) = σ1s2 σ*1s2, σ2s2 σ*2s2, σpx2 π 2py2 2π* 2py2π*2py2 2pz2 σ* 2px2 B.O = 1/2 (10 - 10) = 0 Ne2 cannot exist because its bond order is zero. Molecular Orbital Diagrams - Every Science The procedure for working out a molecular orbital of a general diatomic molecule is quite simple. We construct molecular orbitals using the available orbitals on Thus for the elements O to Ne, which we shall consider initially, the effects of this new principle are not observed. The MO diagram for these...
Molecular Orbital diagram of NO(nitric oxide) molecule Molecular orbital : A molecule in which all the electrons are paired, is called diamagnetic. | Online Chemistry tutorial IIT, CBSE Chemistry, ICSE Chemistry, engineering and medical chemistry entrance exams Molecular orbital diagram of C2 molecule : Number of electrons in C2 molecule = 12. Energy level diagram for Molecular orbitals - Chemical Bonding and... Relationship between electronic configuration and Molecular behaviour. 1) Stability of molecules in terms of bonding and antibonding electrons. 3) If Nb = Na ,the molecule is again unstable because influence of electrons in the antibonding molecular orbital is greater than the bond influence of... Figure 14: The molecular orbital energy-level diagram for diatomic... The molecular orbital energy- level diagram that results is constructed by putting the molecular orbitals in order of increasing number of internuclear nodal planes, the orbital with no such nodal plane lying at lowest energy and the orbital with nodal planes between all the atoms lying at highest energy. (PDF) Inorganic Chemistry by Miessler ~ 5th Edition ... This book is ideal for who want to use a strong molecular-orbital approach to explain structure and reactivity in inorganic chemistry. . × Close Log In. Log in with Facebook Log in with Google. or. Email. Password. Remember me on this computer. or reset password. Enter the email address you signed up with and we'll email you a reset link. ...
CHAPTER 5: MOLECULAR ORBITALS energy level diagram is similar to that of NO (Problem 5.7) without the antibonding π* electron. b. The bond order is three, with no unpaired electrons.29 pages Determine the bond order from the molecular orbital diagram ... Problem: Determine the bond order from the molecular orbital diagram of Ne2. Does the bond order calculated agree with what you would draw for Lewis ...Mar 23, 20201 answer · Top answer: We are asked to determine the bond order from the molecular orbital diagram of Ne2 and to check whether the calculated bond order agrees with the ... 8.4 Molecular Orbital Theory - Chemistry 2e | OpenStax The filled molecular orbital diagram shows the number of electrons in both bonding and antibonding molecular orbitals. However, we can predict that the Be2 molecule and the Ne2 molecule would not be stable. We can see this by a consideration of the molecular electron configurations (Table 8.3). N2+ Mo Diagram - schematron.org Feb 03, 2019 · sorry about that not being a molecular orbital diagram i saw orbital and immediately thought electron configuration to save confusion, could. There are two MO diagrams you need to memorize for diatoms (N2, O2, Ne2, etc) . One is for the elements up to Nitrogen. The other is for AFTER nitrogen.
Molecular Orbital Diagram of O2, F2, and Ne2 Molecules. - YouTube MO Diagram of heteronuclear diatomic Molecules - Chemical Bonding & Molecular Structures - Inorganic. Molecular Orbital (MO) Diagram of Polyatomic molecules Beryllium dihydride (BeH2) and Water (H2O).
Molecular Orbital Theory - Chemistry: Atoms First 2e This is the molecular orbital diagram for the homonuclear diatomic showing the molecular orbitals of the valence shell only. However, we can predict that the Be2 molecule and the Ne2 molecule would not be stable. We can see this by a consideration of the molecular electron configurations ((Figure)).
CHapter 11 Flashcards | Quizlet The valence molecular orbital diagram for the cation f2+ is shown. which of the following options correctly interpret this diagram? Why do the energies of the molecular orbitals formed for species of O2, F2, and Ne2 differ from those of the molecular orbitals for other period 2 elements?
Unlocking surface octahedral tilt in two-dimensional ... Jan 10, 2022 · Using n = 4 RPP as an example, the redshifted narrow emission peak (NE2, full width at half maximum, FWHM ~19 meV, Fig. 4b inset) at 665 nm appears just alongside the main exciton peak (NE1, FWHM ...
Solved Draw the molecular orbital diagram for Ne2+ and | Chegg.com If 2p orbitals on an atom are all the same energy, why do they form molecular orbitals of different engergies when theu mix?
MO Diagrams | Molecular Orbital Diagram Maker A bare molecular orbital diagram is presented and you must drag the correct orbitals and labels onto the diagram. The diagram is then completed by filling the energy levels with the correct number of electrons. The following molecules are currently available: Molecules of the First Row
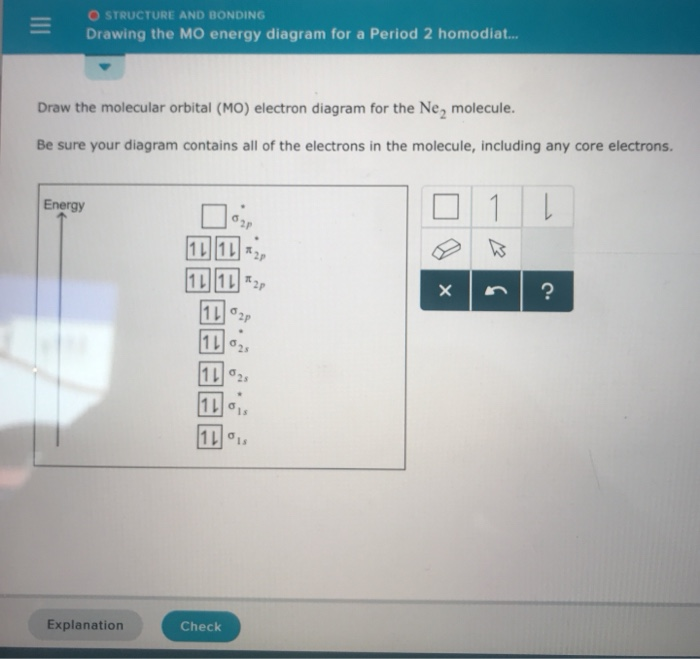
Draw out the molecular orbital diagram for Ne2, starting with ... Calculate the bond order of Ne2 and determine if you would expect this molecule to exist. Then write out the electron configuration for the molecule, and ...4 answers · Top answer: the students welcome here in this question. We have a neon molecule is so we had to ...
Draw and explain the molecular orbital diagram of Ne2 . On ... Molecular orbital diagram of. Explanation: Neon atom has 10 electrons and its electronic configuration is . As the bond order value for molecule is zero, it is unstable and cannot exist. The molecular orbital diagram of hypothetical molecule is given in the attachment.
Molecular Orbital Theory: Explanation, Illustrations and... - Embibe Molecular Orbital Theory: To simplify things, we will consider the interaction of the orbitals containing valence electrons to create molecular orbitals. Have you ever thought about how sigma and pi bonds are formed? What is the difference between diamagnetic and paramagnetic behaviour?
Tutorial on Chemical Bonding, Part 8 of 10 (Molecular orbitals) The molecular orbital model is by far the most productive of the various models of chemical bonding, and serves as the basis for most quantiative calculations, including those that Construct a "molecular orbital diagram" of the kind shown in this lesson for a simple diatomic molecule, and indicate...
Molecular orbital diagram - Wikipedia A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular.
(PDF) Complete Solutions Manual GENERAL ... - Academia.edu Academia.edu is a platform for academics to share research papers.
Molecular orbital diagram — Wikipedia Republished // WIKI 2 A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular.[1][2][3] A fundamental principle of these theories is that...
Chemistry: The Central Science (14th Edition ... 9.7. Molecular Orbitals Molecular Orbitals of the Hydrogen Molecule Bond Order 9.8. Bonding in Period 2 Diatomic Molecules Molecular Orbitals for Li2 and Be2 Molecular Orbitals from 2p Atomic Orbitals Electron Configurations for B2 through Ne2 Electron Configurations and Molecular Properties Heteronuclear Diatomic Molecules Chapter Summary and ...
Why does the molecular orbital diagram for Be2+ consist of... For complete MO diagrams, you use the total number of electrons. The 1s electrons of O2, N2, etc. are used to fill up the sigma(1s) and sigma(1s) Remember, valence electrons are those which do not represent a noble-gas-like-state. the 1s Orbital is full (2 electrons), so the Be2+ configuration is the...
Molecular Orbital Diagrams simplified | by Megan A. Lim | Medium Drawing molecular orbital diagrams is one of the trickier concepts in chemistry. The first major step is understanding the difference between two major Valence Bond Theory proposes that electrons are localized between two atoms. On the other hand, Molecular Orbital Theory visions the electrons of a...
Molecular Orbital Theory: Energy level diagram for molecular orbitals Molecular orbital theory was put forward by Hund and Mullikan in 1932. This theory is modern This energy diagram for the molecular orbitals is shown in Fig.1 However, experimental evidence MOs is valid for molecules or ions like O2, O (super oxide ion), O22-(peroxide ion), F2 and Ne2 (hypothetical).
What is the molecular orbital diagram for B_2? | Socratic Before we can draw a molecular orbital diagram for B₂, we must find the in-phase and out-of-phase overlap combinations for boron's atomic orbitals.
Why does the Ne2 molecule not exist using molecular orbital theory? Molecular orbital confuguration of Ne2 is. The orbital diagram for a diatomic molecule is. To find the bond order, add the 15 electrons in the molecular orbitals (the blue-colored energy levels in the diagram) one at a time until you have used them up.
Introduction to Inorganic Chemistry/Molecular Orbital Theory... Valence bond (VB) theory gave us a qualitative picture of chemical bonding, which was useful for predicting the shapes of molecules, bond strengths, etc. It fails to describe some bonding situations accurately because it ignores the wave nature of the electrons.
Chem 59-250: Molecular Orbital Theory | PDF | Molecular Orbital Molecular Orbital Theory. MO diagrams for other heteronuclear diatomics are formed in exactly the same way as that of H-F or those of the homonuclear Molecular Orbital Theory Polyatomic molecules: The bonding in H3+ For the linear cation, the MO diagram would then be: Chem 59-250.
PDF Molecular Orbitals in | 9-2 Molecular Orbital Energy Level Diagrams Figure 9-2 Molecular orbital (MO) diagram for the combination of the 1s atomic orbitals on two identical atoms (at the left) to form two MOs. Molecular orbital calculations indicate, however, that for O2, F2, and hypothetical Ne2 molecules, the 2p orbital is lower in energy than the 2p orbitals...
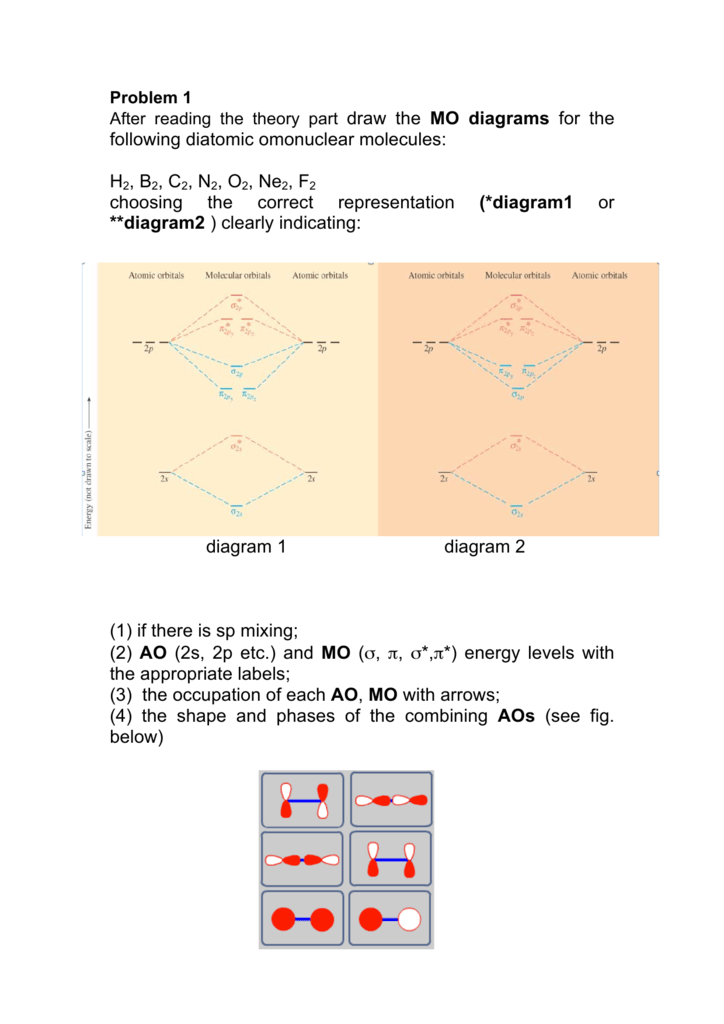
following diatomic omonuclear molecules: H2, B2, C2, N2, O2 ... After reading the theory part draw the MO diagrams for the following diatomic omonuclear molecules: ... Ne2. F2 electron configuration. N. OF. OCCUPIED. MO.13 pages
Molecular Orbital Theory This molecular orbital model can be used to explain why He2 molecules don't exist. Combining a pair of helium atoms with 1s2 electron configurations would The molecular orbital diagram for an O2 molecule would therefore ignore the 1s electrons on both oxygen atoms and concentrate on the...
1000 Solved Problems in Classical Physics - Kamal - Studylib Biblioteca en línea. Materiales de aprendizaje gratuitos. 1000 Solved Problems in Classical Physics Ahmad A. Kamal 1000 Solved Problems in Classical Physics An Exercise Book 123 Dr. Ahmad A. Kamal Silversprings Lane 425 75094 …
PDF Microsoft PowerPoint - An introduction to Molecular Orbital Theory.ppt... - MO diagrams for Inorganic complexes. • It is a waste of both the lecturers and students time if the tutorial to ends up being a lecture covering questions. 5. An introduction to Molecular Orbital Theory.




















0 Response to "38 Molecular Orbital Diagram For Ne2"
Post a Comment