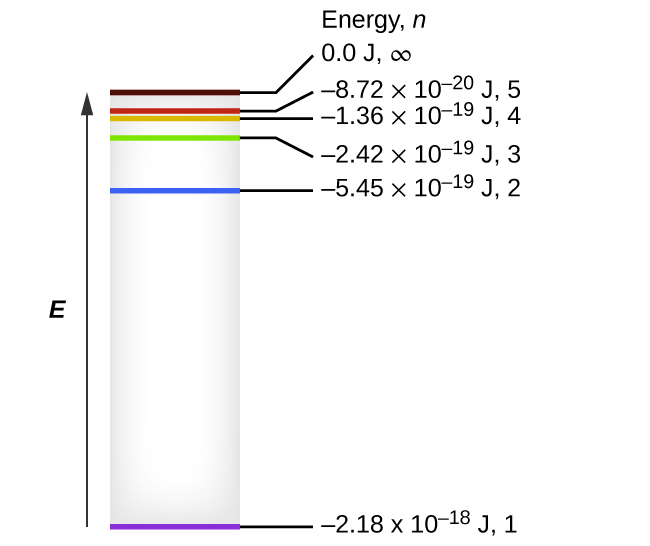
37 the following is a diagram of energy states and transitions in the hydrogen atom.
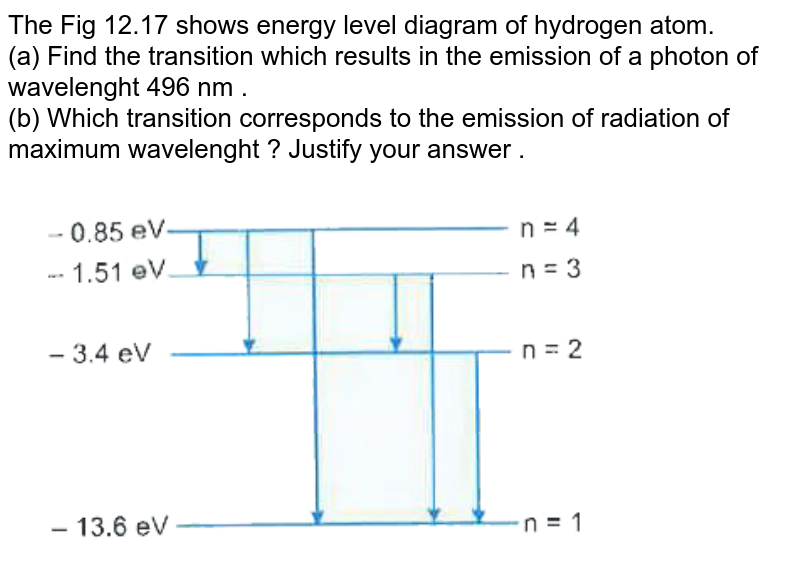
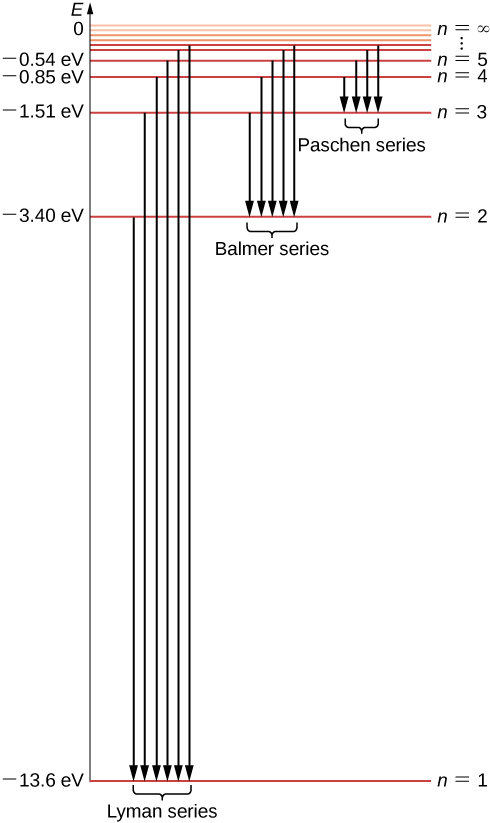
Generally, d-block metals from group 8, 9, and 10 of the periodic table have low overpotentials for hydrogen production. These metals fall in the proximity of the optimal of the Volcano plot (Figure 6.4), of exchange current density (i o) for certain material, and the hydrogen atom absorption energy (ΔE H). The energy of this emitted photon is exactly equal to the difference in atomic energy levels between the initial and final states. In this example, the emitted photon would have 5 units of energy. Because each type of atom has its own unique set of energy levels, each type of atom will emit light with a different set of energies.
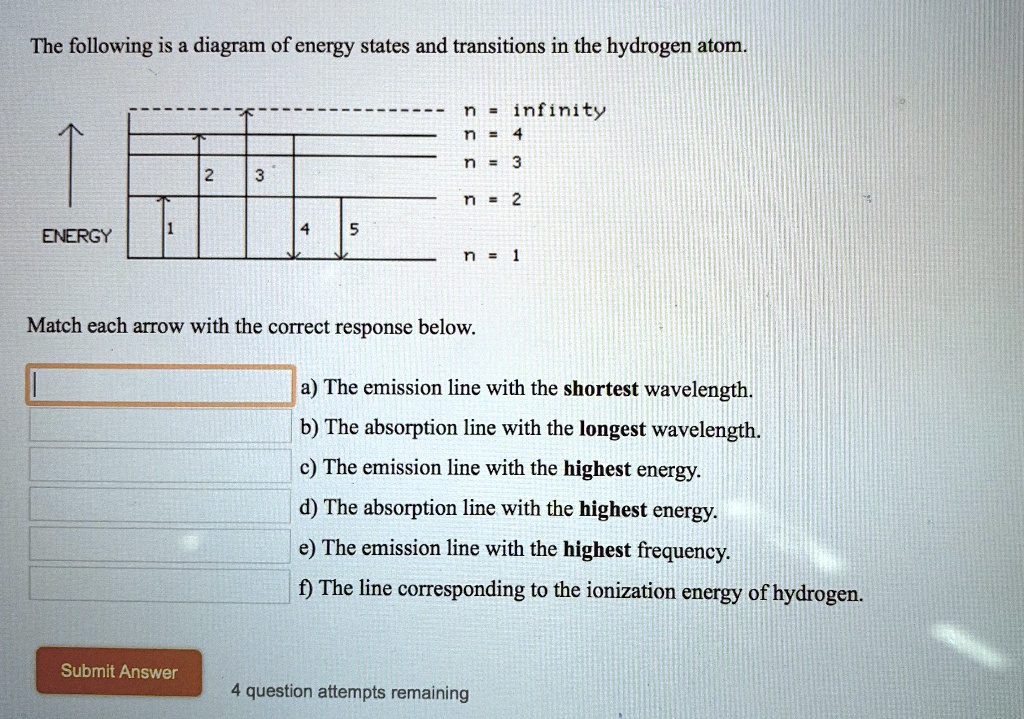
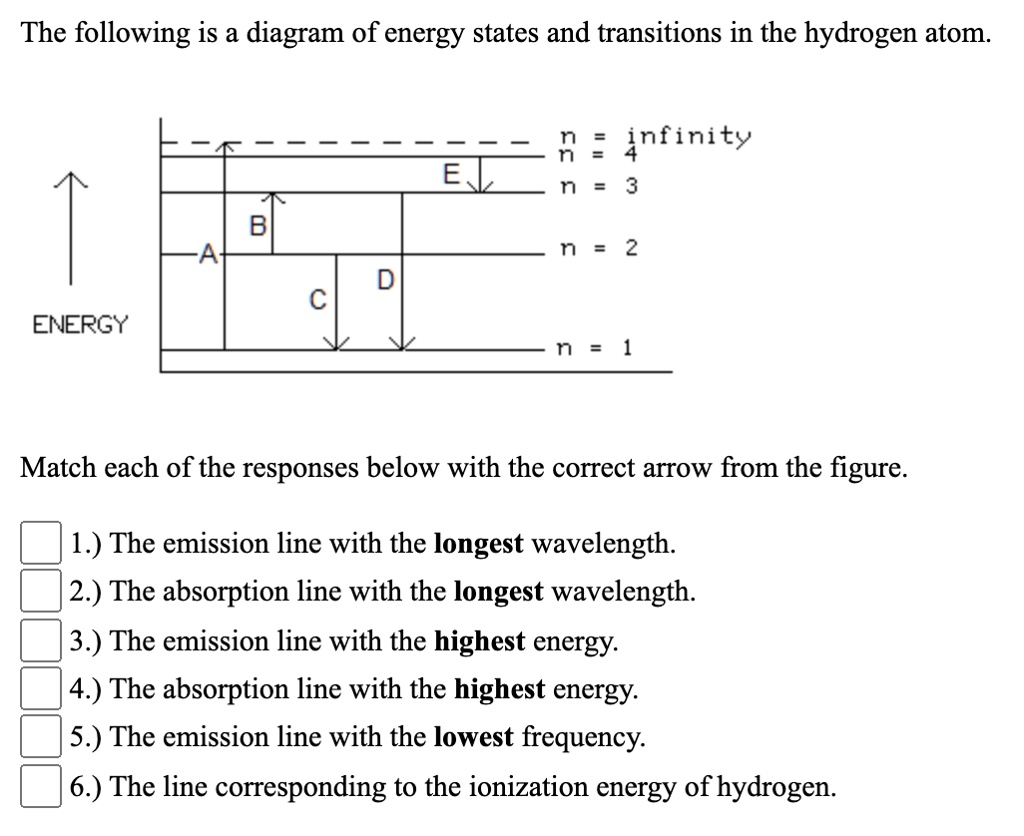
The following is a diagram of energy states and transitions in the hydrogen atom. Match each arrow with the correct response below. a) The emission line with the longest wavelength. b) The absorption line with the shortest wavelength. c) The emission line with the lowest energy. d) The absorption line with the highest energy. e) The emission line with the highest frequency.
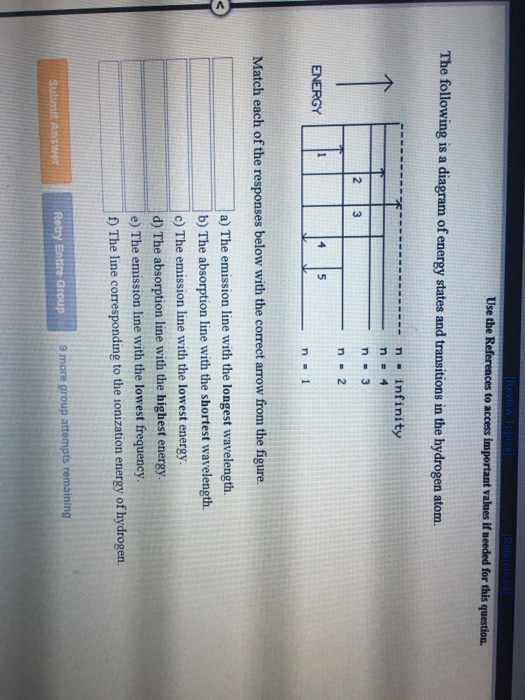
The following is a diagram of energy states and transitions in the hydrogen atom.
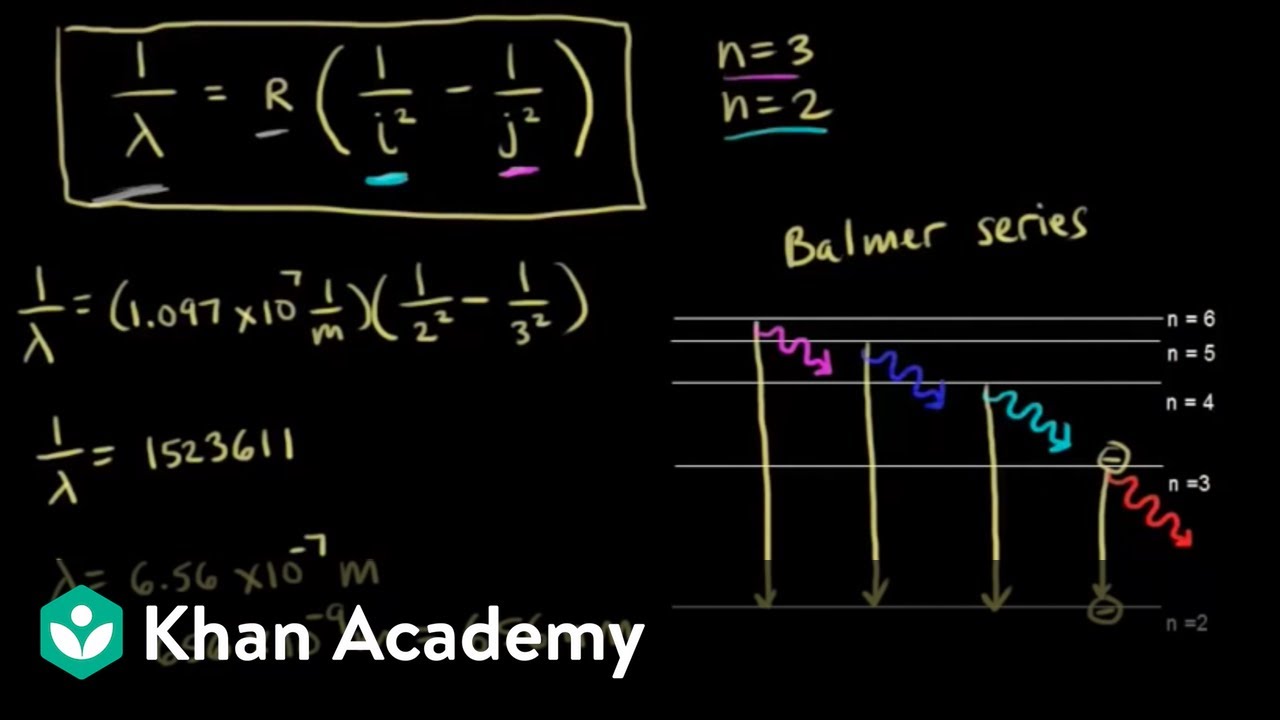
Oct 11, 2021 · The image below shows an energy diagram showing possible transitions between the first seven energy levels in the hydrogen atom. Each series of transitions is named after the scientist who ... Energy bands consisting of a large number of closely spaced energy levels exist in crystalline materials. The bands can be thought of as the collection of the individual energy levels of electrons surrounding each atom. The wavefunctions of the individual electrons, however, overlap with those of electrons confined to neighboring atoms. Transcribed image text: The following is a diagram of energy states and transitions in the hydrogen atom. n. Infinity n = 4 n - 3 2 3 n - 2 1 4 ENERGY 5 n-1 Match each of the responses below with the correct arrow from the figure. a) The emission line with the longest wavelength. b) The absorption line with the shortest wavelength. c) The emission line with the highest energy.
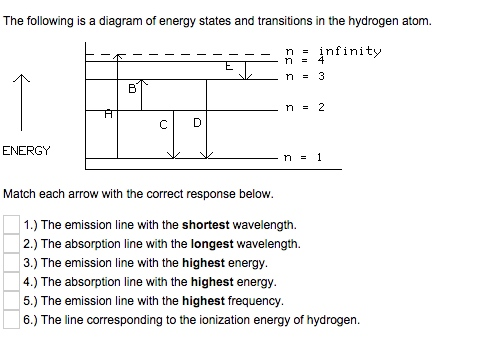
The following is a diagram of energy states and transitions in the hydrogen atom.. An energy-level diagram plots energy vertically and is useful in visualizing the energy states of a system and the transitions between them. This diagram is for the hydrogen-atom electrons, showing a transition between two orbits having energies E 4 and E 2 . Chemistry questions and answers. The following is a diagram of energy states and transitions in the hydrogen atom. Infinity 4 - ח E n = 3 B A n = 2 C ENERGY Match each of the responses below with the correct arrow from the figure. 1.) The emission line with the longest wavelength. 2.) The absorption line with the longest wavelength. 3.) On the other hand, when a hydrogen atom has the energy $-3A$, it is a spin-zero particle. (Remember, what we are saying is only strictly true for infinitesimal magnetic fields.) So we can group the states of hydrogen in zero magnetic field this way: \begin{alignat}{2} & \left. The following is a diagram of energy states and transitions in the hydrogen atom. : Anfinity FI n 1 B A n. 2 D C ENERGY Match each of the responses below with the correct arrow from the figure. 1.) The emission line with the longest wavelength. 2.) The absorption line with the shortest wavelength. 3.) The emission line with the highest energy. 4.)
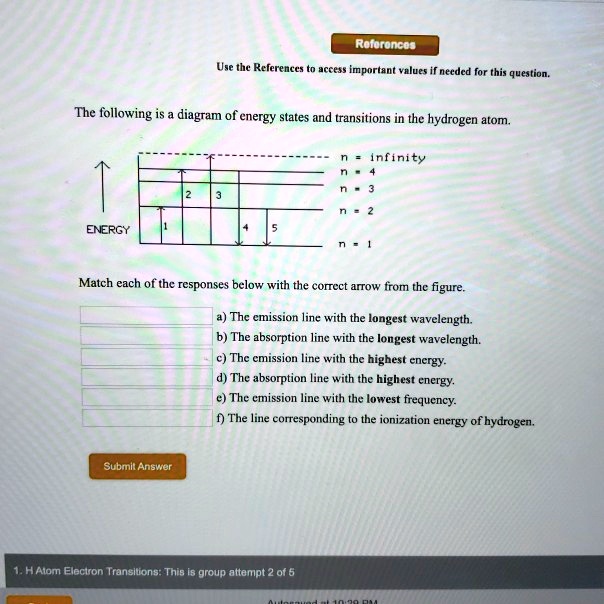
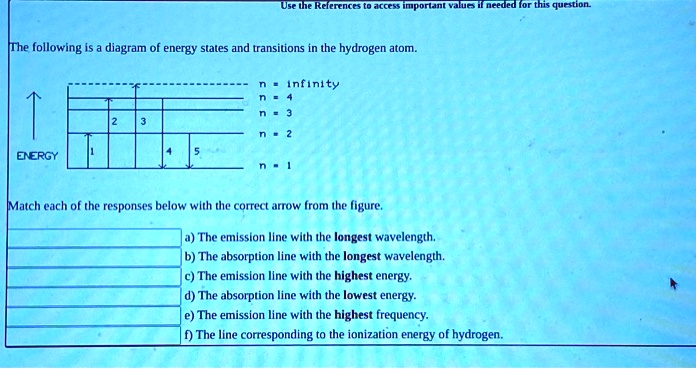
Chemistry questions and answers. The following is a diagram of energy states and transitions in the hydrogen atom. Match each arrow with the correct response below. The emission line with the longest wavelength. The absorption line with the longest wavelength. The emission line with the highest energy. The absorption line with the highest energy. Jun 05, 2019 · From the above energy diagram, you can easily identify which transition has more energy gap and requires more energy for the transition. So totally we have 4 possible electronic transitions with energy requirements for each transition in the following order. σ → σ* > n → σ* > π → π* > π→ π* Note: Transitions of σ → π* and π ... Science Chemistry Q&A Library The following is a diagram of energy states and transitions in the hydrogen atom. n = infinity 4 n = 3 3 n = 2 ENERGY 4 n = 1 Match each arrow with the correct response below. a) The emission line with the shortest wavelength. b) The absorption line with the shortest wavelength. c) The emission line with the lowest energy. Science. Chemistry. Chemistry questions and answers. The following is a diagram of energy states and transitions in the hydrogen atom. Match each of the responses below with the correct arrow from the figure. 1.) The emission line with the longest wavelength._____ 2.) The absorption line with the. Question: The following is a diagram of energy ...
Chemistry. Chemistry questions and answers. The following is a diagram of energy states and transitions in the hydrogen atom. Match each of the responses below with the correct arrow from the figure. fill in the blank ____ 1.) The emission line with the longest wavelength. fill in the blank ____ 2.) The absorption line with the longest ... The following is a diagram of energy states and transitions in the hydrogen atom. n = infinity n = 4 n = 3 2 3 n = 2 5 ENERGY n = 1 Match each of the responses below with the correct arrow from the figure. a) The emission line with the longest wavelength. b) The absorption line with the shortest wavelength. c) The emission line with the lowest ... The Bohr model gives almost exact results only for a system where two charged points orbit each other at speeds much less than that of light. This not only involves one-electron systems such as the hydrogen atom, singly ionized helium, and doubly ionized lithium, but it includes positronium and Rydberg states of any atom where one electron is far away from everything … An atom is the smallest unit of ordinary matter that forms a chemical element.Every solid, liquid, gas, and plasma is composed of neutral or ionized atoms. Atoms are extremely small, typically around 100 picometers across. They are so small that accurately predicting their behavior using classical physics—as if they were tennis balls, for example—is not possible due to quantum …
Jun 25, 2020 · The following areas are covered; porous materials, liquid hydrogen carriers, complex hydrides, intermetallic hydrides, electrochemical storage of energy, thermal energy storage, hydrogen energy systems and an outlook is presented for future prospects and research on hydrogen-based energy storage.
The followmg is a diagram of energy states and transitions in the hydrogen atom. n = n = Infinity EJ, n B A n = 2 D ENERGY n = 1 Match each of the responses below with the correct arrow from the figure. 4 1 5 1.) The emission line with the longest wavelength 2.) The absorption line with the shortest wavelength. 3.)
Transcribed image text: The following is a diagram of energy states and transitions in the hydrogen atom. n. Infinity n = 4 n - 3 2 3 n - 2 1 4 ENERGY 5 n-1 Match each of the responses below with the correct arrow from the figure. a) The emission line with the longest wavelength. b) The absorption line with the shortest wavelength. c) The emission line with the highest energy.
Energy bands consisting of a large number of closely spaced energy levels exist in crystalline materials. The bands can be thought of as the collection of the individual energy levels of electrons surrounding each atom. The wavefunctions of the individual electrons, however, overlap with those of electrons confined to neighboring atoms.
Oct 11, 2021 · The image below shows an energy diagram showing possible transitions between the first seven energy levels in the hydrogen atom. Each series of transitions is named after the scientist who ...
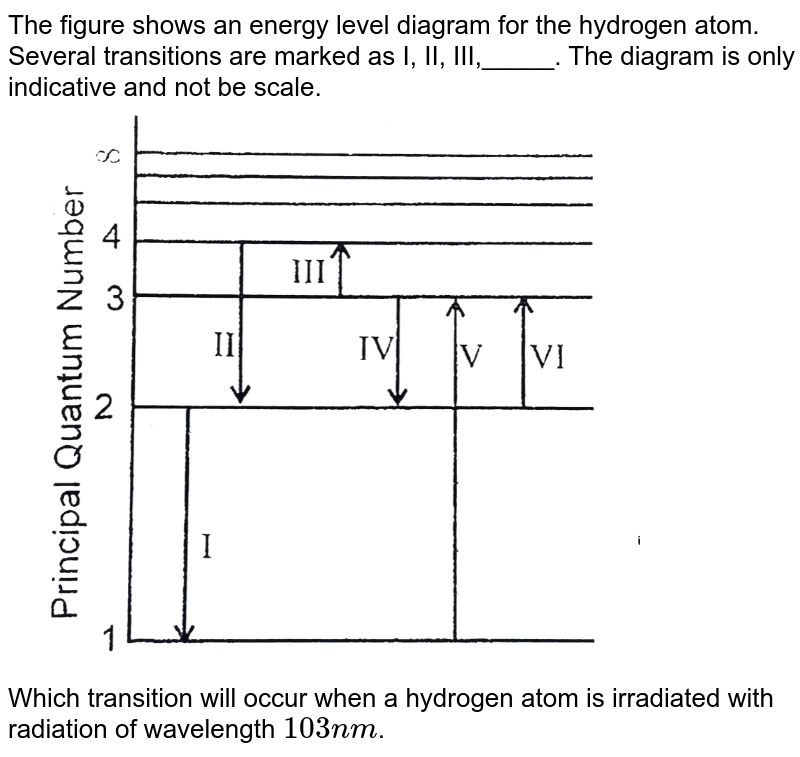













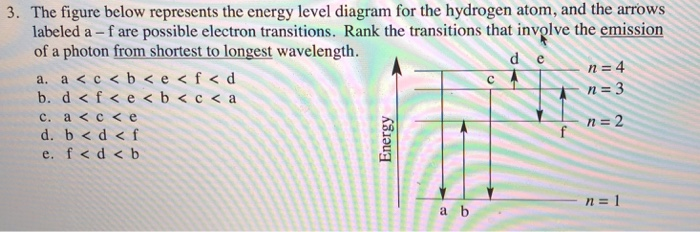






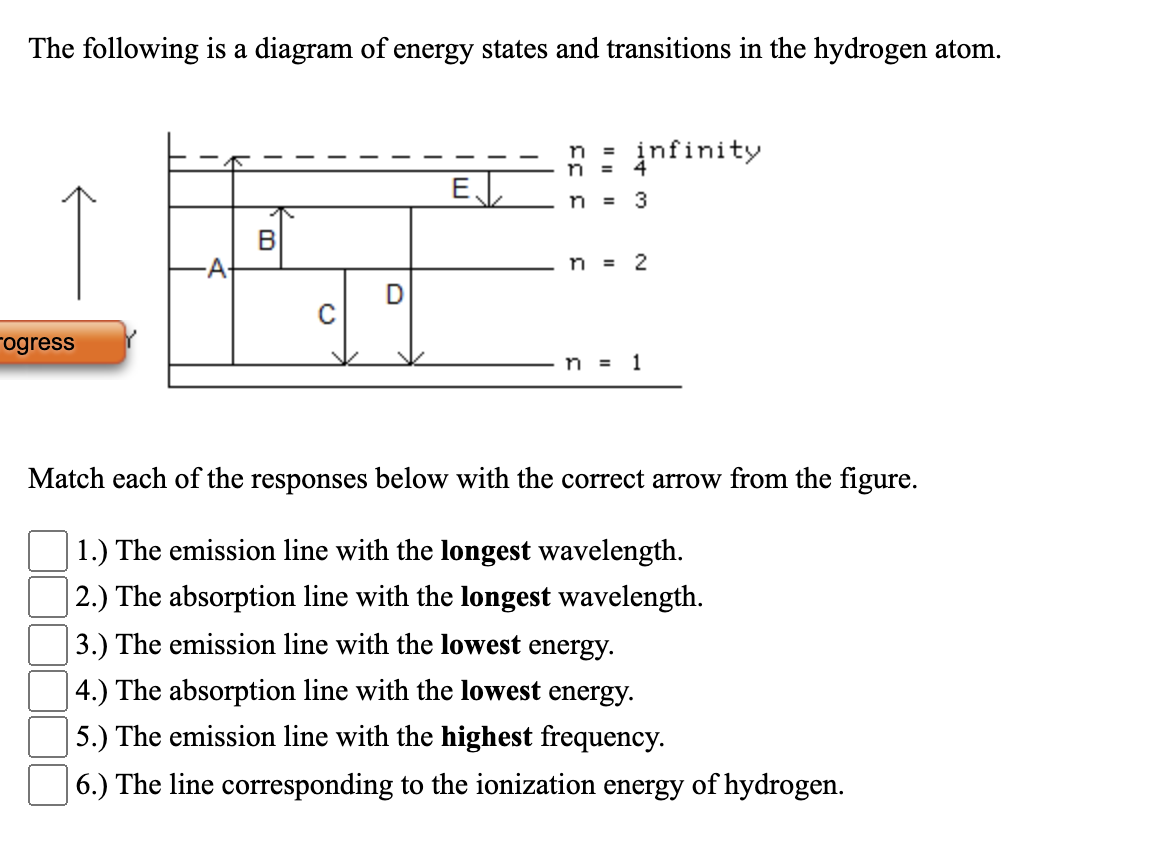
0 Response to "37 the following is a diagram of energy states and transitions in the hydrogen atom."
Post a Comment