37 create the atomic orbital diagram for chlorine
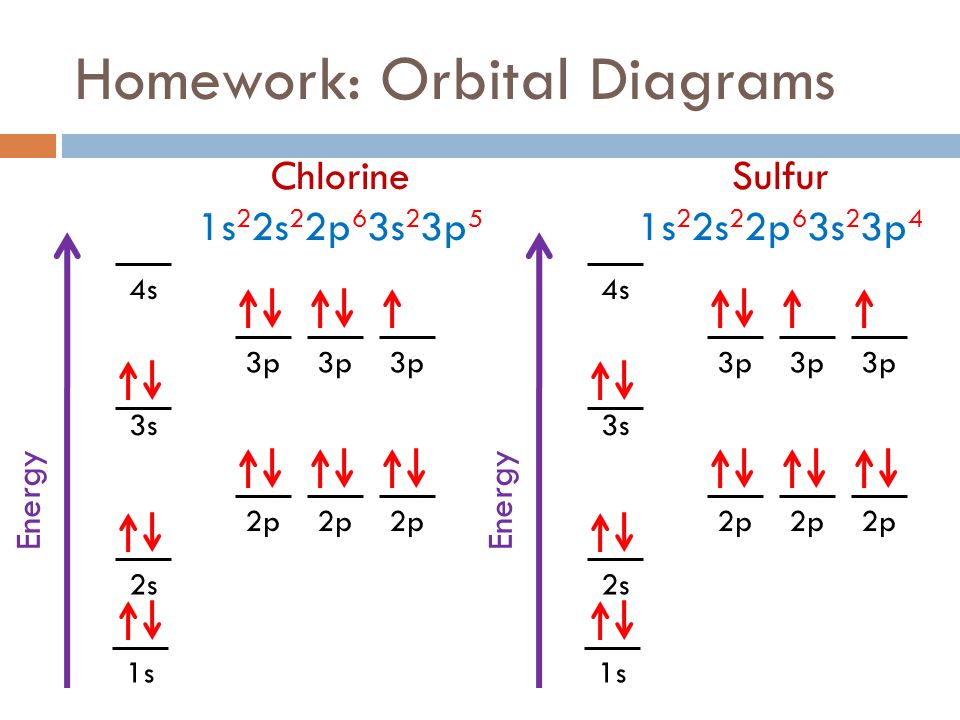
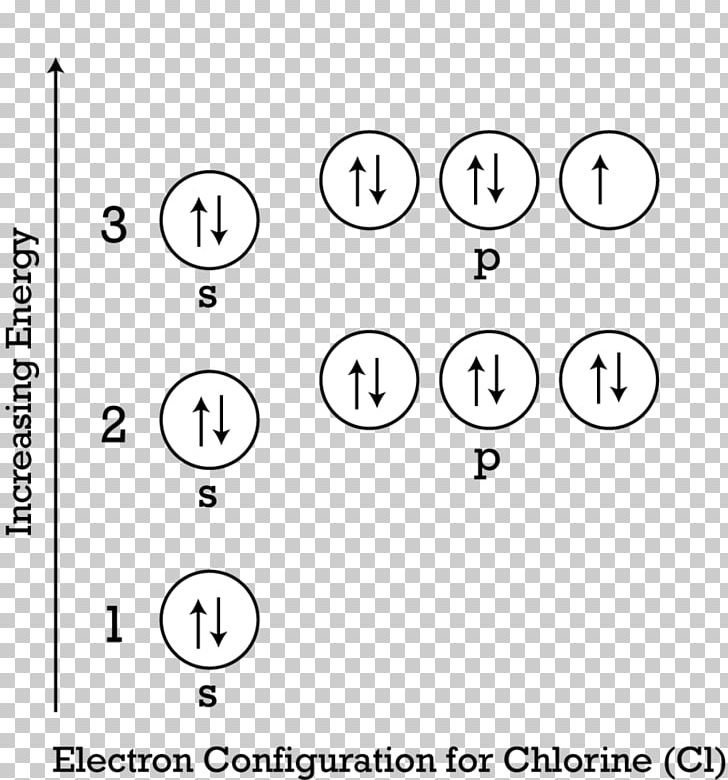
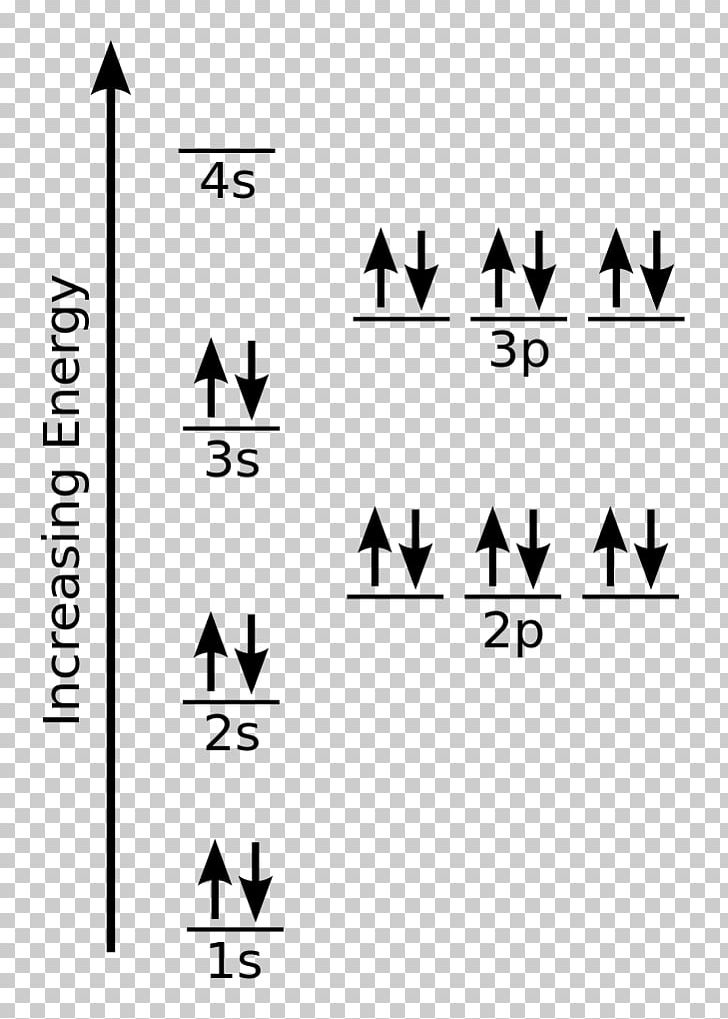
Electron Configuration for Chlorine (Cl) - UMD When we write the configuration we'll put all 17 electrons in orbitals around the nucleus of the Chlorine atom. In writing the electron configuration for Chlorine the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Chlorine go in the 2s orbital. Orbital Diagrams, Rules & Principles Quiz - Quizizz Q. What is the Pauli Exclusion Principle? answer choices. An atomic orbital can only hold a maximum of 2 electrons, each with opposite spins. An atomic orbital can hold a minimum of 6 electrons, each with opposite spins. An atomic orbital can hold a maximum of 6 electrons, each with the same spin.
What is orbital diagram for chlorine? - Answers An orbital is a region of space that an electron can exist in. For the diagram you start with the 1 s orbital and then 2s, 2p, and so on. Each orbital can hold 2 electrons and each arrow ...
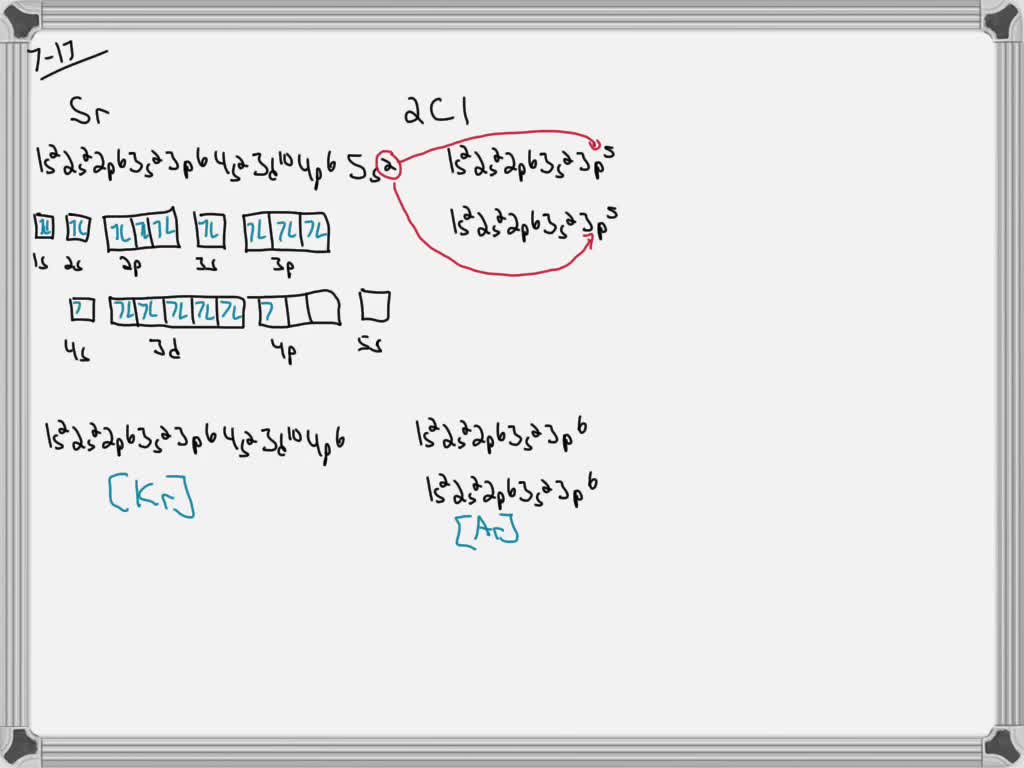
Create the atomic orbital diagram for chlorine
How to Do Orbital Diagrams - Sciencing Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics. Hybrid orbitals - 1 Sketch out a diagram illustrating how the plots of atomic s- and p- orbital wave functions give rise to a pair of hybrid orbitals. Draw "orbital box" diagrams showing how combinations of an atomic s orbital and various numbers of p orbitals create sp, sp 2, and sp 3 hybrid orbitals. PDF Orbital diagram of chlorine 17 - Weebly The orbital 1s at the bottom of the diagram is the orbital with electrons of lower energy. Energy increases as we advance to orbitals 2s and then 2p, 3s and 3p, showing that the increasing n- value has more influence on energy than increasing the l-value for small atoms.
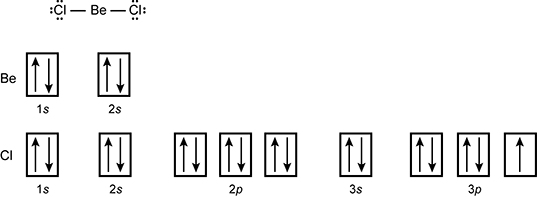
Create the atomic orbital diagram for chlorine. SOLVED:Assume the energy level diagram shown in Figure 9 ... So, um, now we're going to create our atomic orbital. Um, this is not our atomic orbital, but our molecular orbital diagram, Um, using our atomic orbital's. So first will fill up the atomic orbital's for chlorine or chloride. Remember, we have seven valence electrons, so we're going to start at the two s level and then start adding from there ... 5.2 Hybrid Atomic Orbitals - Chemistry: Atoms First | OpenStax Figure 5.9 This orbital energy-level diagram shows the sp hybridized orbitals on Be in the linear BeCl 2 molecule. Each of the two sp hybrid orbitals holds one electron and is thus half filled and available for bonding via overlap with a Cl 3p orbital. Chlorine, atomic structure - Stock Image - C013/1530 ... Chlorine (Cl). Diagram of the nuclear composition and electron configuration of an atom of chlorine-35 (atomic number: 17), the most common isotope of this element. The nucleus consists of 17 protons (red) and 18 neutrons (blue). 17 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). Orbital Diagram of All Elements (Diagrams given Inside) Atomic no. Orbital Diagram of All Elements Diagrams; 1: Orbital diagram of Hydrogen (H) 2: Orbital diagram of Helium (He) 3: Orbital diagram of Lithium (Li) 4: Orbital diagram of Beryllium (Be) 5: Orbital diagram of Boron (B) 6: Orbital diagram of Carbon (C) 7: Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram ...
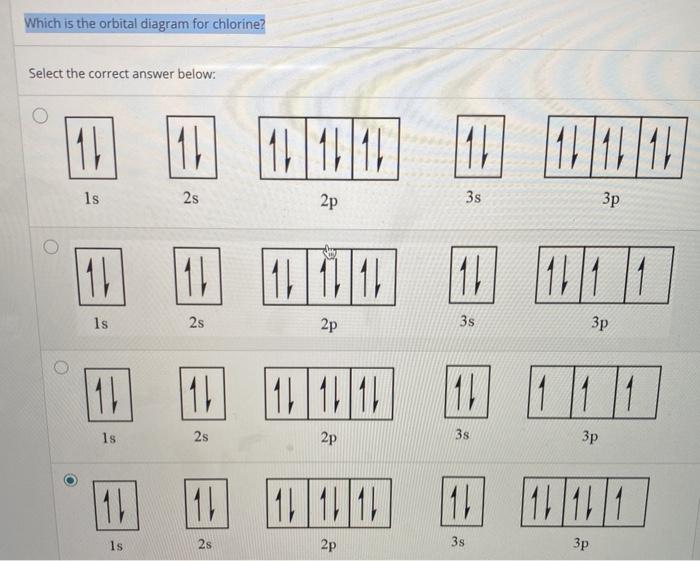
OneClass: valence electrons chlorine Create the atomic orbital diagram for chlorine. In a chlorine atom, which subshells contain valence electrons? Show transcribed image text Create the atomic orbital diagram for chlorine. Draw the atomic orbital diagram for sulfur... | Clutch Prep Our videos prepare you to succeed in your college classes. Let us help you simplify your studying. If you are having trouble with Chemistry, Organic, Physics, Calculus, or Statistics, we got your back! Our videos will help you understand concepts, solve your homework, and do great on your exams. 7.5 Hybrid Atomic Orbitals - Chemistry Fundamentals Figure 7.5.4. This orbital energy-level diagram shows the sp hybridized orbitals on Be in the linear [latex]\ce{BeCl2}[/latex] molecule. Each of the two sp hybrid orbitals holds one electron and is thus half filled and available for bonding via overlap with a [latex]\ce{Cl}[/latex] 3p orbital. identify the element corresponding to the orbital diagram ... Create the atomic orbital diagram for chlorine. In a chlorine atom, which subshells contain valence electrons? Show transcribed image text Create the atomic orbital diagram for chlorine.
Give orbital diagram of the following:magnesium chloride, Give orbital diagram of the following:magnesium chloride, Give orbital diagram of the following: magnesium chloride, Solved Create the atomic orbital diagram for chlorine. In ... Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. 100% (15 ratings) Transcribed image text: Create the atomic orbital diagram for chlorine. In a chlorine atom, which subshells contain valence electrons? PDF Chapter 11 required and write a partial orbital diagram. PROBLEM: Use partial orbital diagrams to describe how mixing of the atomic orbitals of the central atom(s) leads to hybrid orbitals in each of the following: (a) Methanol, CH. 3. OH (b) Sulfur tetrafluoride, SF. 4 (a) CH. 3. OH. The electron- group arrangement is tetrahedral around both the C and ... Sodium (Na): How to write the Orbital Diagram, Electron ... This video shows how to draw the orbital diagram of Sodium (Na). It also shows how to write the electron configuration of Sodium (Na) and the shorthand nobl...

apply use electron configurations orbital notation and electron dot structures to represent the form
Molecular Orbital Diagram For Cl2 - schematron.org Molecular Orbital Diagram - Cl2, Br2, I2 3s & 3p and higher atomic orbitals are not so widely separated in energy and allow significant mixing (hybridization) to occur. This mixing causes the inversion of the σσand πmolecular orbitals' energy. σσσ ππ σ* π* 3,4,5 p 3, 4,5 s σ* σ 3,4,5 s 3,4,5 p Interhalogens Br Br F F Br F F F F.
Chem4Kids.com: Chlorine: Orbital and Bonding Info Chlorine atomic orbital and chemical bonding information. There are also tutorials on the first thirty-six elements of the periodic table. Check out the blackboard. That box on the left has all of the information you need to know about one element. It tells you the mass of one atom, how many ...
Chlorine(Cl) electron configuration and orbital diagram Chlorine ground-state electron configuration and orbital diagram When chlorine atoms are excited, then chlorine atoms absorb energy. As a result, an electron in the 3p y sub-orbital jumps to the 3d xy sub-orbital. The d-orbital has five sub-orbitals. The sub-orbitals are d xy, d yz, d zx, d x2-y2 and d z2.
Cl Atomic Orbitals - uwosh.edu Cl Atomic Orbitals. On the left in the table below (you may need to scroll down) is a window with a green dot in it. The green dot represents the location of a chlorine nucleus (significantly enlarged so that you can see it). On the right hand side are four pull-down menus from which you can choose an orbital to display.
Sodium(Na) electron configuration and orbital diagram Sodium(Na) is the 11th element in the periodic table and its symbol is 'Na'. This article gives an idea about the electron configuration of sodium and orbital diagram, period and groups, valency and valence electrons of sodium, bond formation, compound formation, application of different principles.Hopefully, after reading this article you will know in detail about this.
Draw the atomic orbital diagram for chlorine. Q. Create the atomic orbital diagram for nitrogen. Q. Write the corresponding electron configuration for the following pictorial representation.Give the full electron configuration (do not use the noble...
PDF Electron Configurations and Orbital Diagrams key Write the electron configuration (full, and in core notation) for the following ions: 1.-1Br +3 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 [Kr], [Ar] 3d 10 4s 2 4p 6 2. Sr +2 8. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4s [Kr], [Ar] 3d 10 4s 2 4p 6 3. +2Se-2 9. 1s 2 2s2 2p6 3s 2 3p 6 3d 10 4s 2 4p 6 [Kr], [Ar] 3d 10 4s 2 4p 6 4.
Molecular Orbital Energy Diagram Cl2 33 Create The Atomic Orbital Diagram For Chlorine - Wiring ... Molecular Orbital Energy Level Diagram Li2 and Li2+ - YouTube 9.7: Molecular Orbitals - Chemistry LibreTexts
PDF Chlorine orbital notation - Weebly Chlorine orbital notation C Predict which structure is preferred based on the formal charge on each atom and its electronegativity relative to the other atoms present. Which electron configuration represents the electrons in an atom of chlorine in an excited state? Chlorine is the second halogen, being a nonmetal in group 17 of the periodic table.
Cl2 Molecular Orbital Diagram Cl2 Molecular Orbital Diagram. A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in . This means that the σ 1 s molecular orbital has a lower energy than either of the hydrogen 1s atomic orbitals.
Solved Create the atomic orbital diagram for a chlorine ... Create the atomic orbital diagram for a chlorine atom. In a chlorine atom, which subshells contain valence electrons ? DDDDD Energy Tools Single orbitals (click to add/remove): 1s 2s 2p 3s 3p 3d 4s 4p 4d 41 5s 5p 5d 5f 6s 6p 6d 6f 7s 7p 7d 74 85 8p 8d 8f All electrons: All orbitals Fill all shells Add all orbitals Empty all shells Remove all ...
PDF Orbital diagram of chlorine 17 - Weebly The orbital 1s at the bottom of the diagram is the orbital with electrons of lower energy. Energy increases as we advance to orbitals 2s and then 2p, 3s and 3p, showing that the increasing n- value has more influence on energy than increasing the l-value for small atoms.
Hybrid orbitals - 1 Sketch out a diagram illustrating how the plots of atomic s- and p- orbital wave functions give rise to a pair of hybrid orbitals. Draw "orbital box" diagrams showing how combinations of an atomic s orbital and various numbers of p orbitals create sp, sp 2, and sp 3 hybrid orbitals.
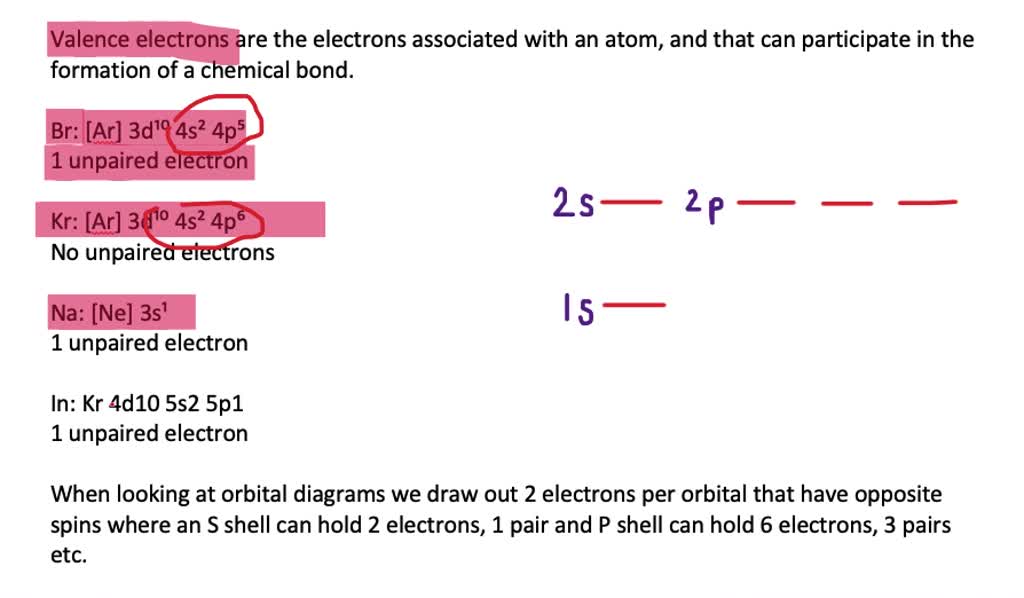
write the electron configuration for a chlorine atom and draw the orbital diagram for chlorine determine the following write the number answer only number of unpaired electrons number of val 35367
How to Do Orbital Diagrams - Sciencing Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics.




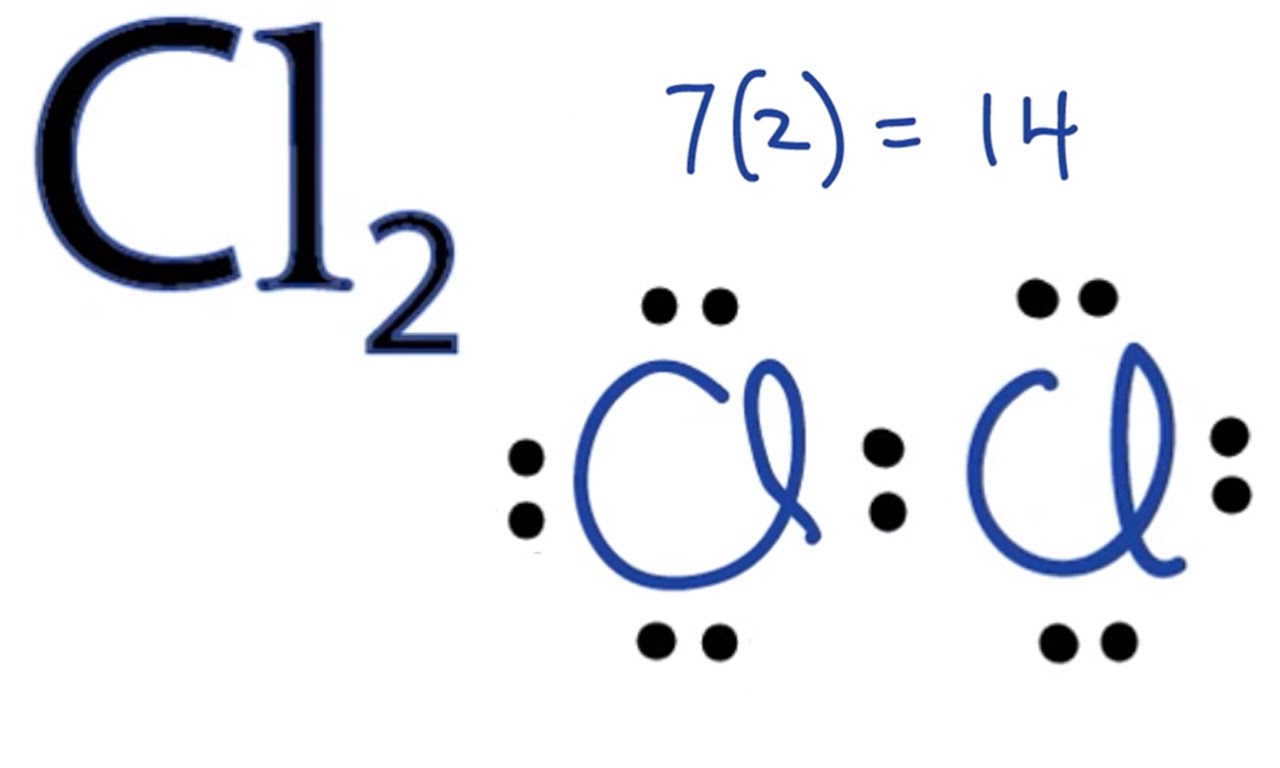























0 Response to "37 create the atomic orbital diagram for chlorine"
Post a Comment