40 mo diagram for h2o
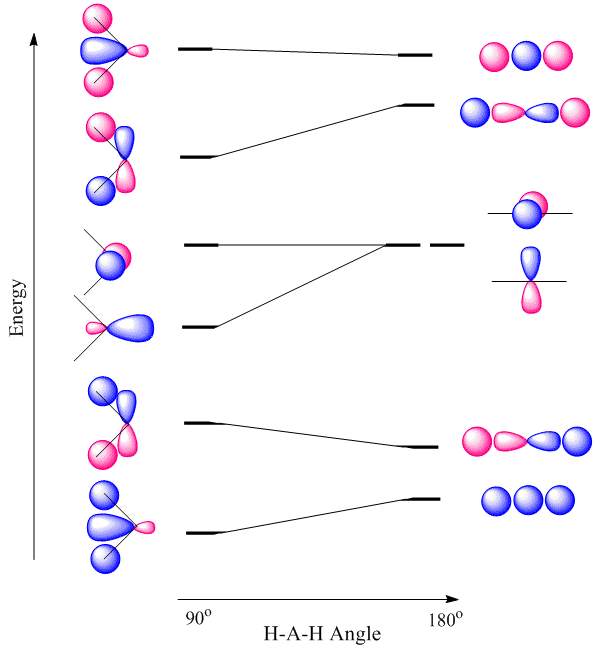
Walsh diagram better overlap of H 1s orbitals the occupied MO lying closest to it governs the geometric preference. a complex MO diagram: B2H6 MO diagrams combine two fragments. Symmetry .. fragment =5e therefor keep up to b2 orbital z x y b1 a1 a1 b2. BH2. Fig. Molecular Orbital Theory - Walsh diagram The Walsh diagram shows what happens to the molecular orbitals for a set of molecules which are related in structure. In this case, the difference is the H-X-H bond angle which decreases from 180 o to 90 o Molecular Orbital Theory - Walsh diagram Water 104.5 ° X H H H O H
The MO Diagram for Water. Revision. • molecular orbitals are combinations of atomic orbitals. • atomic orbitals: o atomic orbitals have a radial and angular.14 pages

Mo diagram for h2o
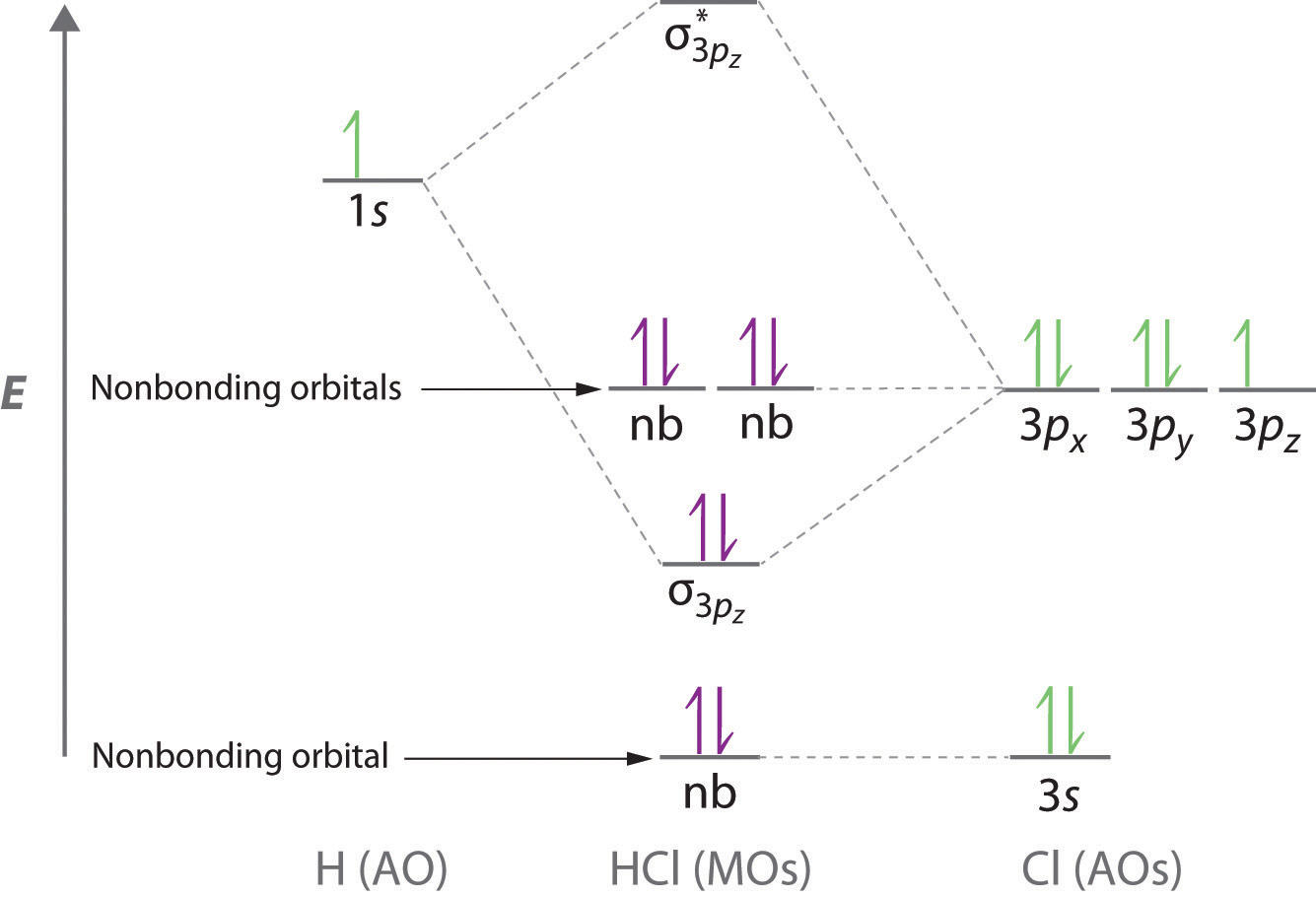
WALSH DIAGRAM. For a linear geometry as AH 2 (H 2 O) tri atomic molecule, the lowest energy value molecular orbital 2σg is formed by s-orbital of A and 1s-orbital of H-atom. On bending the molecule 2a 1 orbital forms a slightly lower orbital in energy than the originally 2σg orbitals, This is due to the fact that 1s orbital of H-atom energy is better with each-other and with p-orbital of A ... MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H–F nb σ σ* Energy H –13.6 eV 1s F –18.6 eV –40.2 eV 2s 2p So H–F has one σ bond and three lone electron pairs on fluorine General Features of MO Diagrams Bonding MOs are found at low energy Non-bonding or lone-pair MOs are at higher energy Antibonding MOs are usually vacant and at high energy Highest occupied MO (HOMO) is nucleophilic/Lewis basic ... Bonding in the Water Molecule Just as in H 2, the two H atoms in C 2v H 2O are equivalent by symmetry The two H ...
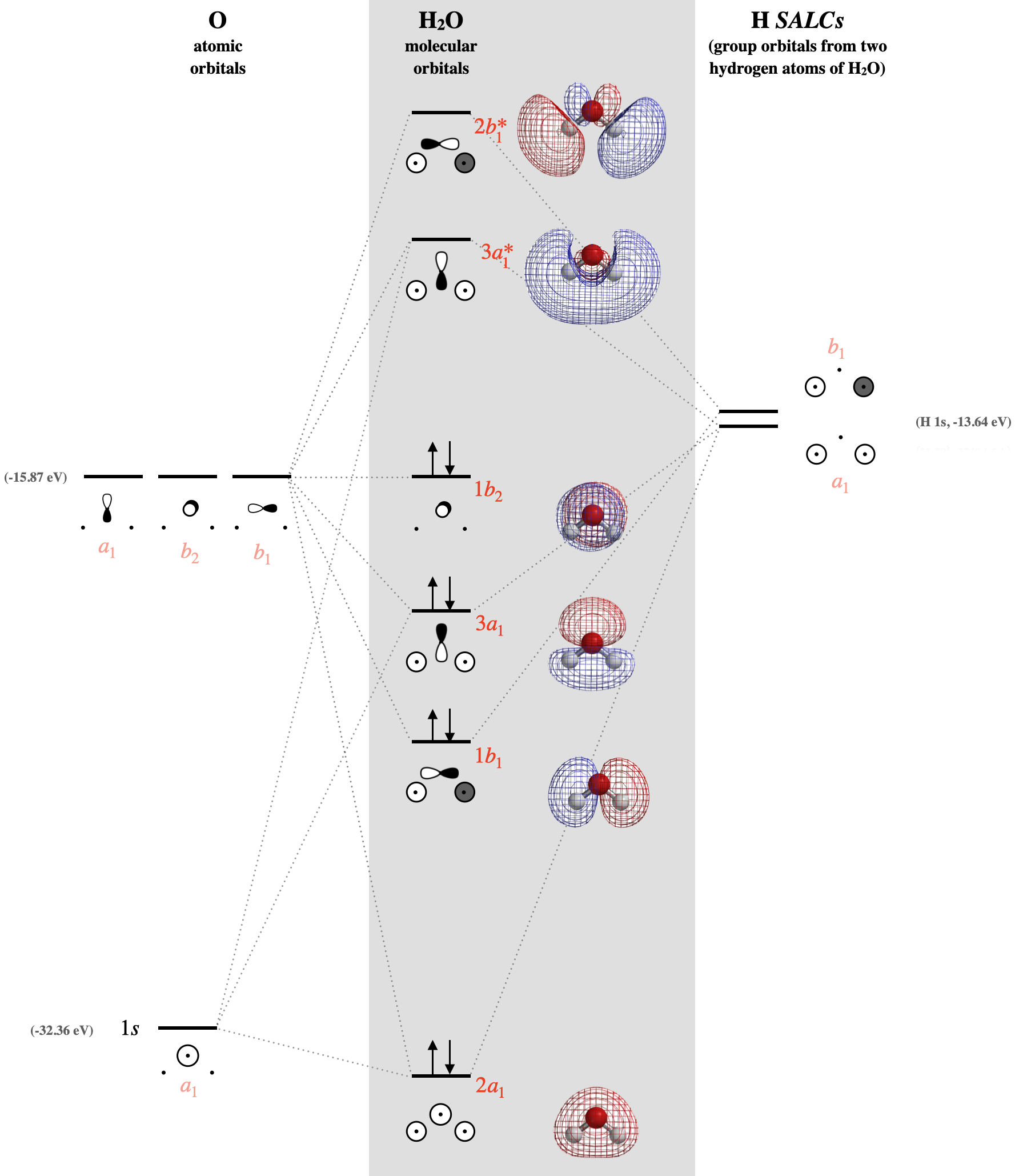
Mo diagram for h2o. the respective electrons of each are displayed in their orbitals (not shown on the diagram but for oxygen you have 6 valence electrons, this constitutes 2x2s electrons, 4x2p electrons) for H 2 it is 2x 1s now effective molecular orbital formation is done by mixing and overlap of similar atomic orbitals. In order to construct an MO diagram for water, we'll take a stepwise approach: first, determine the way in which the hydrogen atoms can combine (in phase or out of phase, as per the standard dihydrogen MO diagram) which will give us a set of symmetry adapted combinations Question: Use the MO diagram for H2O to address the following questions: a) which molecular orbitals are associated with the lone pairs in the H2O molecule? b) are the two lone pairs in H2O equivalent to one another? This problem has been solved! Construct SALCs and the molecular orbital diagram for H\(_2\)O. This is the first example so far that is not a linear molecule. Water is a bent molecule, and so it is important to remeber that interactions of pendant ligands are dependent on their position in space.
H2o Mo Diagram. mo of h2og an advanced molecular orbital diagram of h2o water for the inorganic or physical chemistry student file h2o mo diagramg wikimedia mons english mo diagram of water vectorized simplified and corrected from file diagramme ah2 quantitative calculations show bonding character in both. Hf Molecular Orbital Diagram. H2 Molecular Orbital Diagram MO diagram of dihydrogen Bond breaking in MO diagram The smallest molecule, hydrogen gas exists as dihydrogen (H-H) with a single covalent bond between two hydrogen atoms. As each hydrogen atom has a single 1s atomic orbital for its electron, the bond forms by overlap of these two atomic orbitals. molecular orbital diagram as a non-bonding molecular orbital. 7. There are a total of 6 electrons to add to the molecular orbital diagram, 3 from boron and 1 from each hydrogen atom. sp Hybrid Orbitals in BeH2 1. The Lewis structure shows that the beryllium in BeH 2 makes 2 bonds and has no lone pairs. It is a linear molecule. Chemistry 104 ... Figure 2: The orbital interaction diagram of (H2O)2. Orbital energy levels are represented as solid bars. The bars on the left and right sides correspond to the FOs of the two water monomers; the...
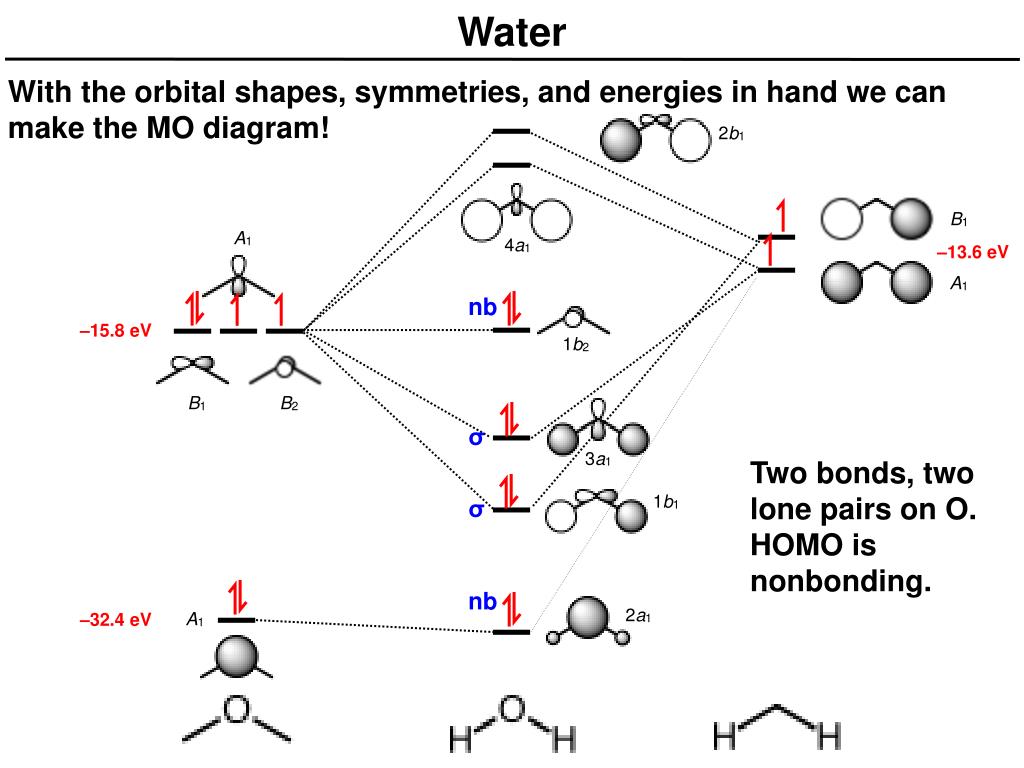
An advanced molecular orbital diagram of H2O (water) for the inorganic or physical chemistry student. Water With the orbital shapes, symmetries, and energies in hand we can make the MO diagram! A1 A1 B1 B2 -15.8 eV -32.4 eV A1 B1 -13.6 eV 2a1 3a1 4a1 1b1 2b1 1b2 nb nb σ σ Two bonds, two lone pairs on O. HOMO is nonbonding. In the simple MO diagram of H 2O, the 2s orbital of oxygen is mixed with the premixed hydrogen orbitals.Jul 31, · Oxygen is sp3 hybridised in H2O molecule. Two hybrid orbitals are occupied by lone pairs and two are used in bonding with Hydrogen atoms. Since lone pairs does not contribute to the geometry of a molecule, therefore H2O has an angular geometry. In the simple MO diagram of H 2O, the 2s orbital of oxygen is mixed with the premixed hydrogen orbitals, forming a new bonding (2a1) and antibonding orbital (4a1). Similarly, the 2p orbital (b1) and the other premixed hydrogen 1s orbitals (b1) are mixed to make bonding orbital 1b1 and antibonding orbital 2b1.
H3O+ Molecular Orbital (MO) Diagram. A molecular orbital diagram of any molecule gives us an idea about the mixing of orbitals in the molecule. Talking about the overlap diagram of H3O+, it is almost similar to H2O but with one electron less and one hydrogen more. Given below is the image of the molecular orbital diagram of H3O+ and also that ...
29.08.2018 · H2O. ↓ ψ1s. ↓. R1sY1s. ↓ Form the molecular orbitals for the basic or first stage MO diagram. • first work. General procedure for simple molecules that contain a central atom: build group orbitals using the outer atoms, then interact the group orbitals. Molecular Orbitals for Water (H2O). H2O molecular orbitals. The five occupied and the lowest three unoccupied molecular …
12.10.2018 · Using group theory to crate a qualitative MO diagram for water
Answer (1 of 3): Oxygen: 1s2, 2s2, 2p4 Hydrogen: 1s1 The bonds are polar-covalent.
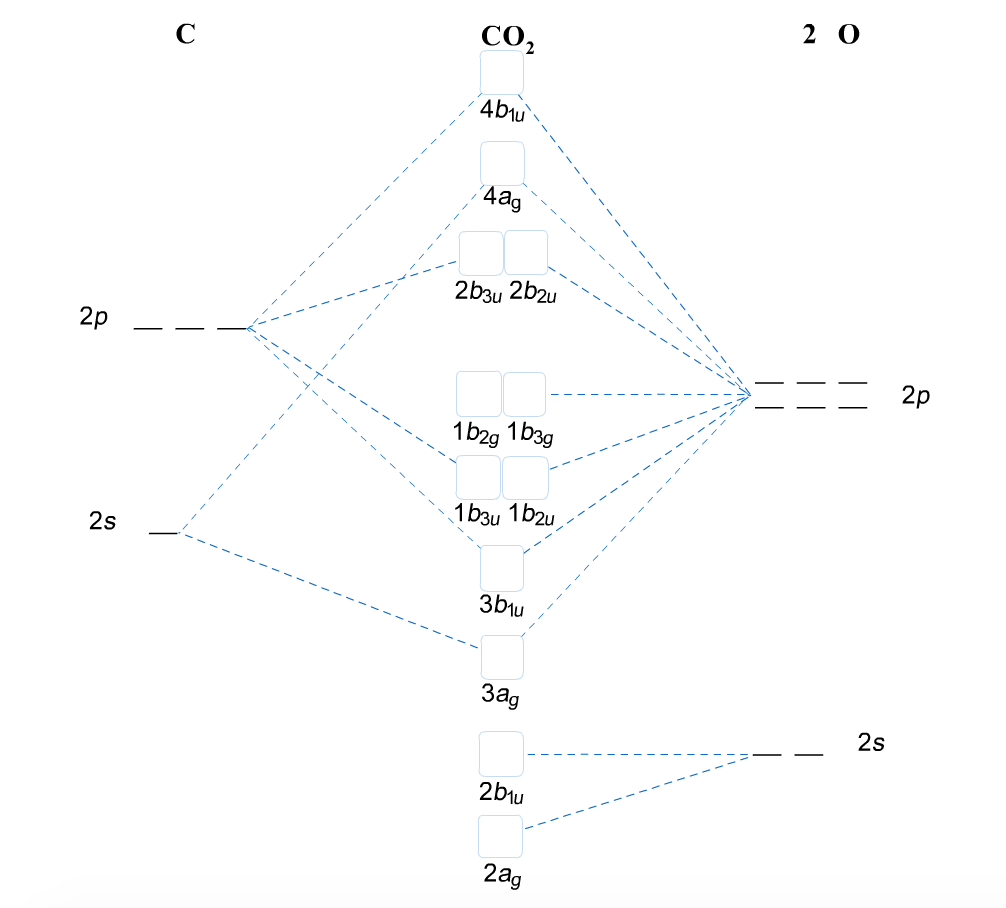
2. The 10-Step approach to making MO diagrams via symmetry considerations 3. Molecular orbital diagram for bent H2O 4. Molecular orbital diagram for linear BeH2 5. Molecular orbital diagram for square planer XeF4 6. Using p-orbitals for σ-bonding: molecular orbital diagram for trigonal planer BF3 1.
Oct 17, 2020 · Description. H2O-MO-Diagram.svg. English: MO diagram of water. Vectorized, simplified and corrected from File:Diagramme AH2.png. Quantitative calculations show bonding character in both the 3a 1 and 2a 1 levels. Date. 21 May 2015. Source. Own work.
sketch the MO diagram for the formation of H3O+ from H+ and H2O; Question: sketch the MO diagram for the formation of H3O+ from H+ and H2O. This problem has been solved! See the answer See the answer See the answer done loading. sketch the MO diagram for the formation of H3O+ from H+ and H2O.
21.10.2019 · Follow me on instagram-https://www.instagram.com/trickychemistrysuman/?hl=enFollow me on facebook page-https://lm.facebook.com/l.php?u=https%3A%2F%2Ffb.me%2F...
A simple MO diagram for water is as shown in the table. MO diagram for water Thus we can see in the center of the diagram the 2 bonding orbitals (containing 4 electrons) the two non-bonding orbitals (containing 4 electrons) and the 2 antibonding orbitals (which are vacant) formed from the basis set of six O(2s), O(2p) (x3) and H(1s) (x2) atomic ...
The bond order of H2O is 2, and that of NH3 is 3, which makes sense, when considering the number of bonds they have. However, if using basic group theory, you get the MO diagrams as presented in this paper: Upon examination, we see that in the case of H2O, there are two orbitals that are strongly bonding, one weakly bonding orbital, and one non ...
Molecular Orbital diagram of water (H2O) The molecular orbital diagram is a pictorial representation of determining chemical bonding between the molecules of a compound. Furthermore, the molecular orbital diagram helps with determining how two sigma bonds have been formed and the effect of the lone pairs on the structure.
We start by making the same H AO combinations we used for H 2 and also for BeH 2. Then we do sp-mixing on O, making one orbital pointed toward the H atoms and one pointed away (which will be mostly non-bonding, like a lone pair). Then we make bonding and anti-bonding combinations of H and O orbitals that match. MO diagram for water.
The highest occupied molecular orbital (HOMO), 1b 1, is predominantly p z 2 in character with no contribution from the hydrogen 1s orbital and mainly contributes to the "lone pair" effects. The 2a 1, 1b 2 and 3a 1 all contribute to the O-H bonds. The two lowest unoccupied molecular orbitals 4a 1 (LUMO) and 2b 2
Download scientific diagram | Molecular orbital diagrams of Cl2, H2O, and Br2. from publication: Theoretical Study of the Potential Energy Surfaces of the Van Der Waals H 2 O−X 2 + (X = Cl or Br ...
General Features of MO Diagrams Bonding MOs are found at low energy Non-bonding or lone-pair MOs are at higher energy Antibonding MOs are usually vacant and at high energy Highest occupied MO (HOMO) is nucleophilic/Lewis basic ... Bonding in the Water Molecule Just as in H 2, the two H atoms in C 2v H 2O are equivalent by symmetry The two H ...
MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H–F nb σ σ* Energy H –13.6 eV 1s F –18.6 eV –40.2 eV 2s 2p So H–F has one σ bond and three lone electron pairs on fluorine
WALSH DIAGRAM. For a linear geometry as AH 2 (H 2 O) tri atomic molecule, the lowest energy value molecular orbital 2σg is formed by s-orbital of A and 1s-orbital of H-atom. On bending the molecule 2a 1 orbital forms a slightly lower orbital in energy than the originally 2σg orbitals, This is due to the fact that 1s orbital of H-atom energy is better with each-other and with p-orbital of A ...























0 Response to "40 mo diagram for h2o"
Post a Comment