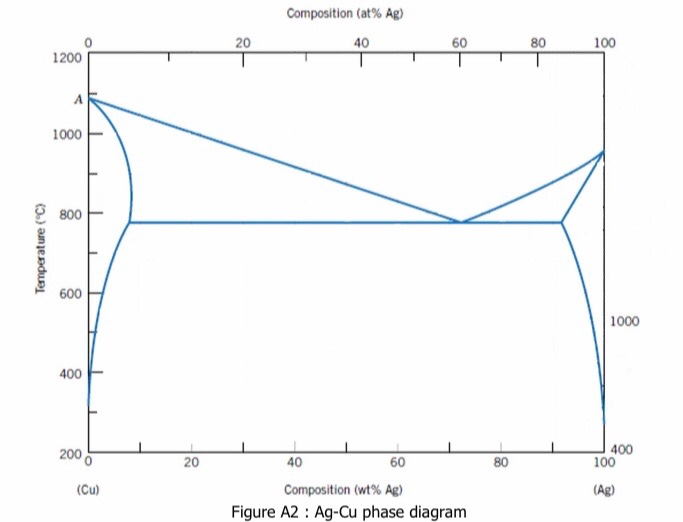
40 ag-cu phase diagram
Janet, The areas on the right and left of that diagram represent the single phase solid areas in the Ag Cu system. What that means is at the ratios and temperatures shown the alloy formed is a single phase solid solution. The reading of phase diagrams is a fairly complex subject and is not one to be condensed to a single email response May 27, 2009 · The phase diagram of the Ag–Cu–In system was investigated using powder X-ray diffraction (XRD), differential scanning calorimetry (DSC) and electron probe microanalysis (EPMA). Two isothermal sections (at 510 and 607 °C) and 15 isopletic sections were studied. The results showed seven ternary peritectics, one ternary eutectic and one ternary metatectic.
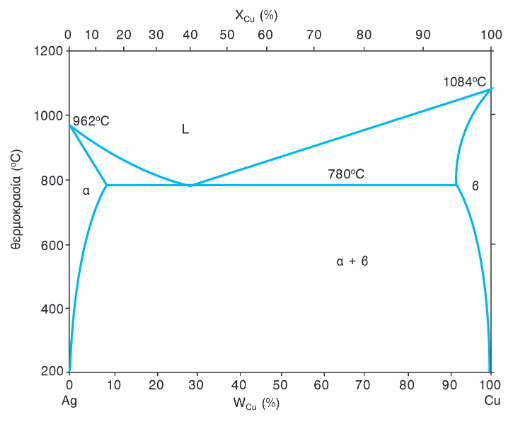
Download scientific diagram | Phase diagram of Cu-Ag from publication: Fabrication, properties and microstructures of high strength and high conductivity copper-silver wires | Research results of ...
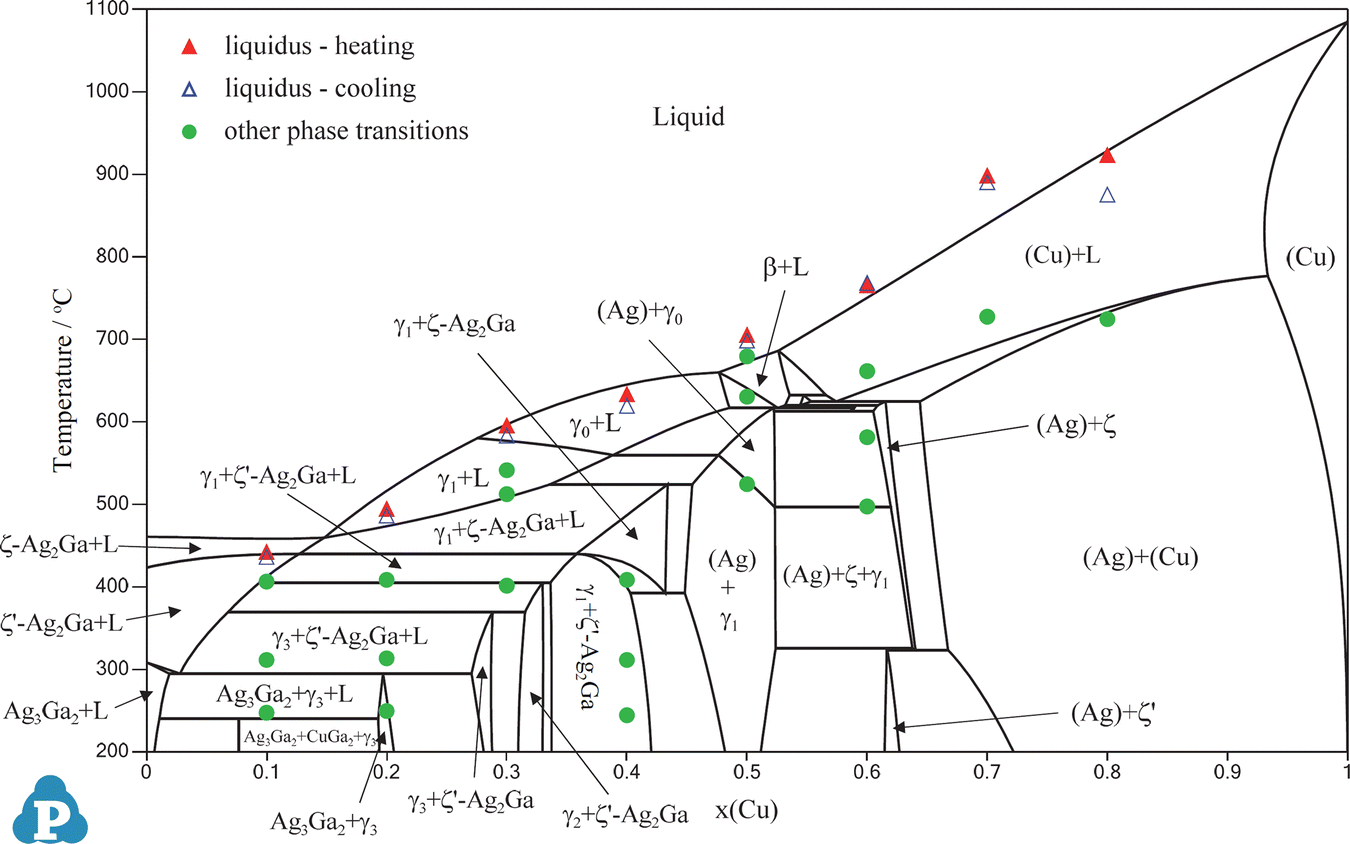
Ag-cu phase diagram
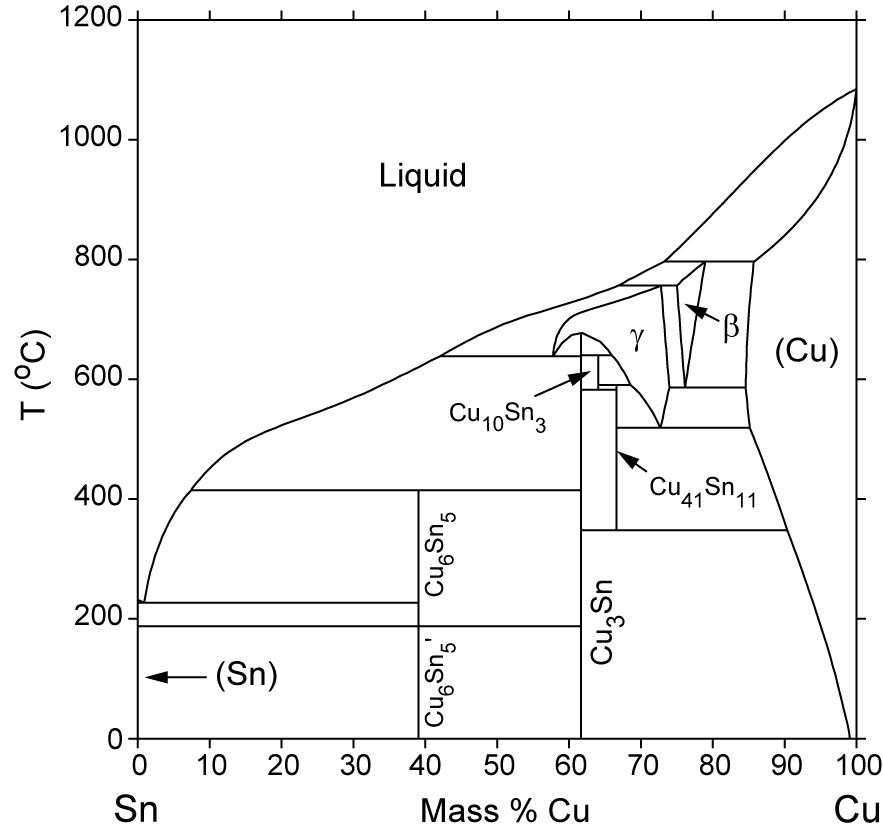
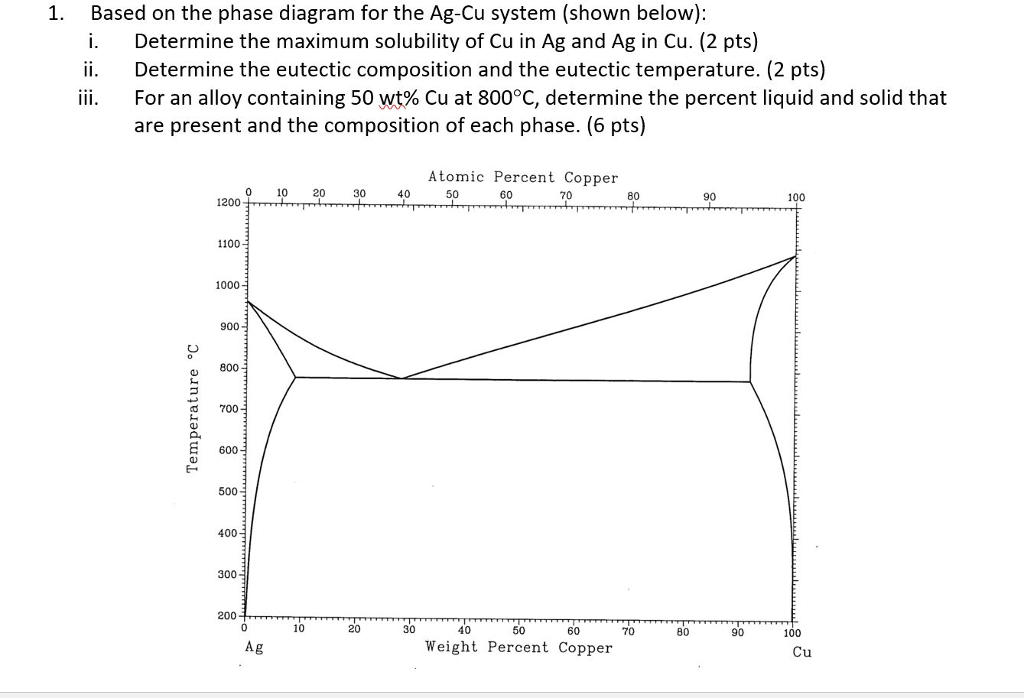
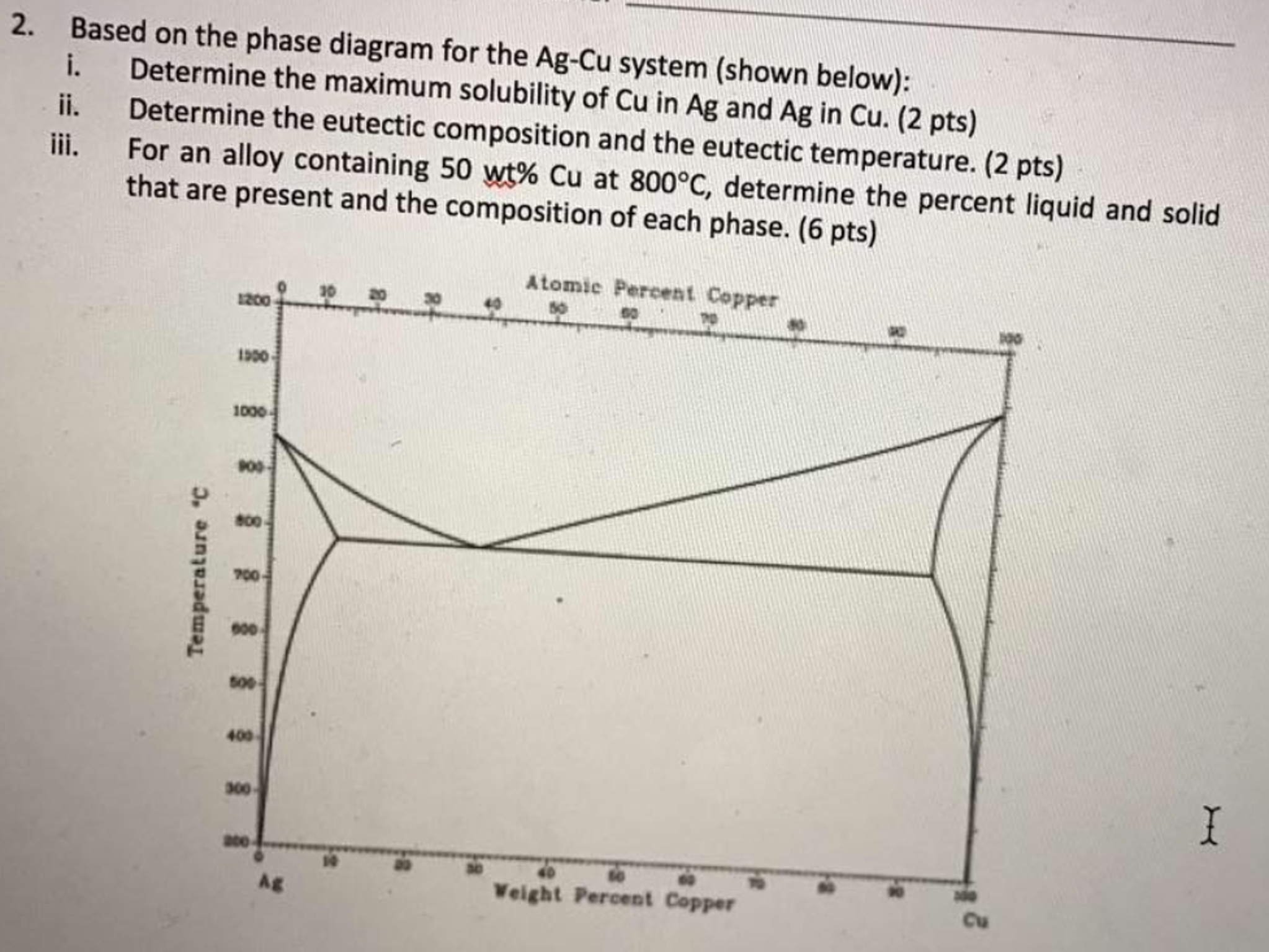
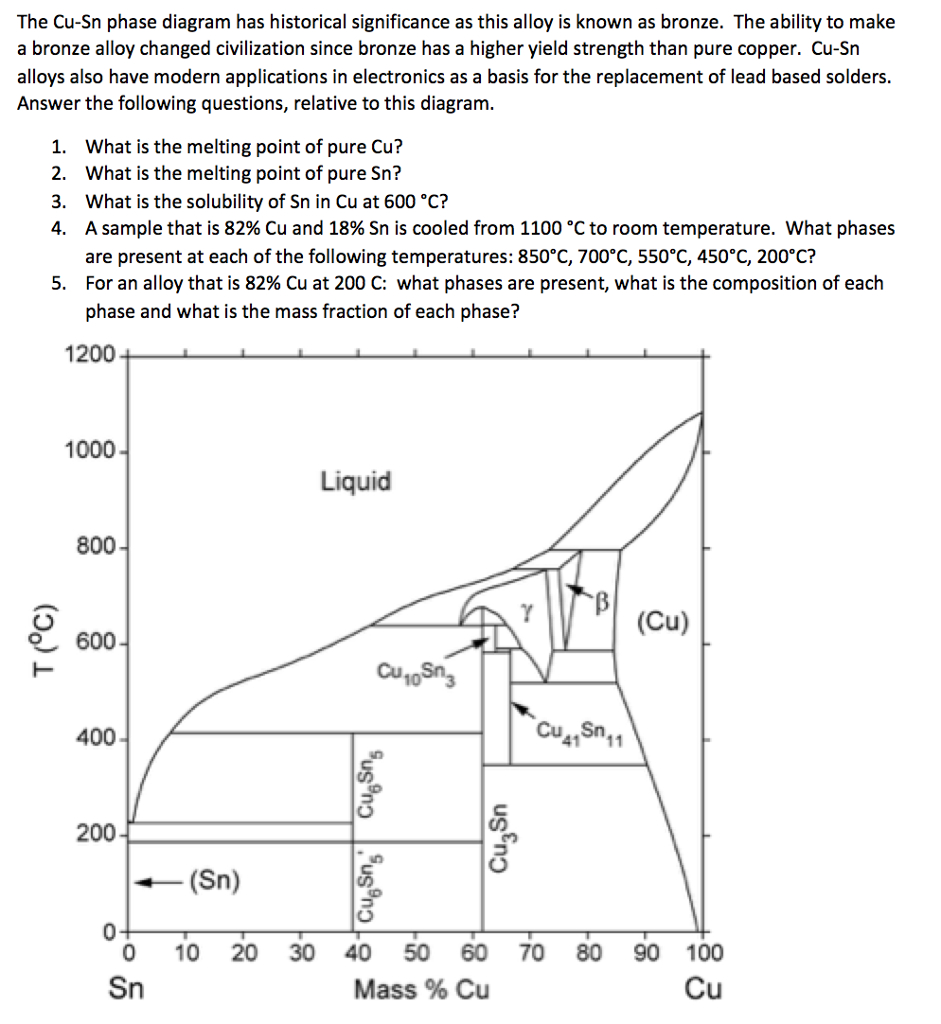
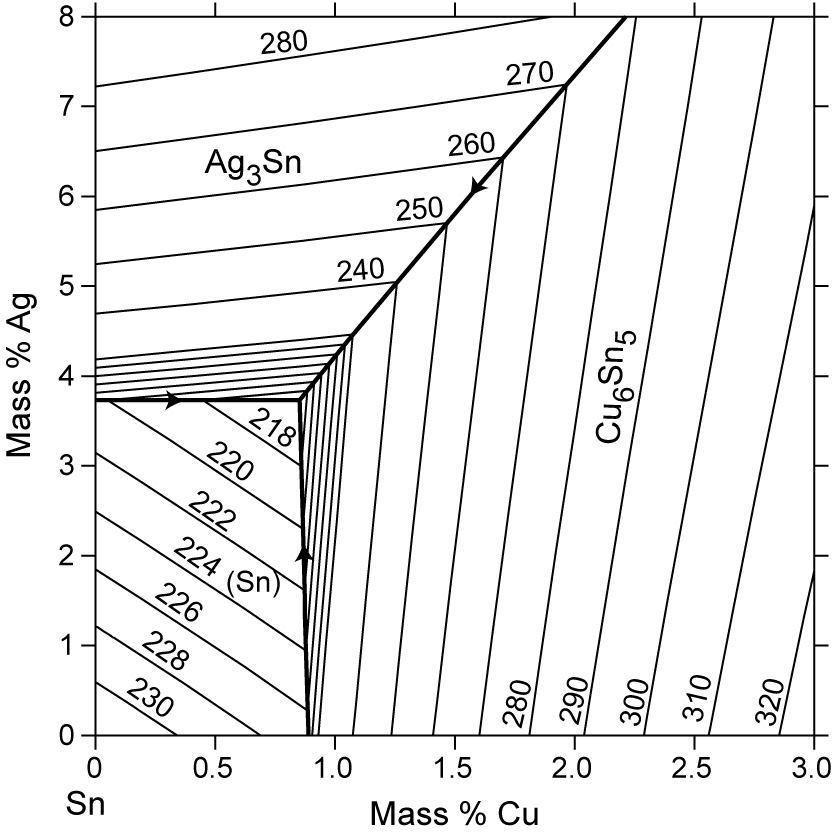
L + Cu 3 Sn -> Ag 3 Sn + Cu 6 Sn 5. 356.2 o C: Liquid: 19.26: 5.86: 74.88: Cu 3 Sn: 0: 61.63: 38.37: Ag 3 Sn: 73.17: 0: 26.83: Cu 6 Sn 5: 0: 39.07: 60.93: L -> Ag 3 Sn + Cu 6 Sn 5 + (Sn) 215.9 o C: Liquid: 3.73: 0.85: 95.42: Ag 3 Sn: 73.17: 0: 26.83: Cu 6 Sn 5: 0: 39.07: 60.93 (Sn) 0.07: 0: 99.93: L + gamma + Cu 3 Sn 677.0 o C. Univariant maximum: Liquid: 7.51: 48.64: 43.83: gamma: 0: 61.63: 38.37: Cu 3 Sn: 0: 61.63: 38.37 Sn-rich alloys in the Sn-Ag-Cu system are being studied for their potential as Pb-free solders. Thus, the location of the ternary eutectic involving L, (Sn), Ag3Sn and Cu6Sn5 phases is of critical interest. Phase diagram data in the Sn-rich corner of the Sn-Ag-Cu system are measured. The ternary eutectic is confirmed to be at a composition of 3.5 The Ag-Cu phase diagram is given in the figure below. According to this phase diagram, what is: The melting temperature of pure Ag The melting temperature of pure Cu The maximum solubility of Cu in Ag@ 5009C The maximum solubility of Ag in Cu at 600°C The eutectic composition The eutectic temperature The phase rich in Cu The phase rich in Ag Composition (at% Ag) 100 2200 20 1200 1000 Liquid 1800 1600 a +L 800 1400 1200 600 1000 200 100 20 60 Composition (wt% Ag)
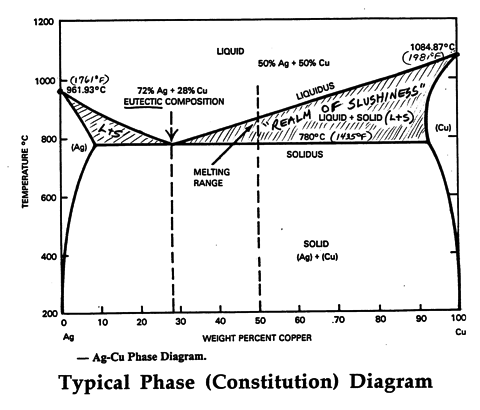
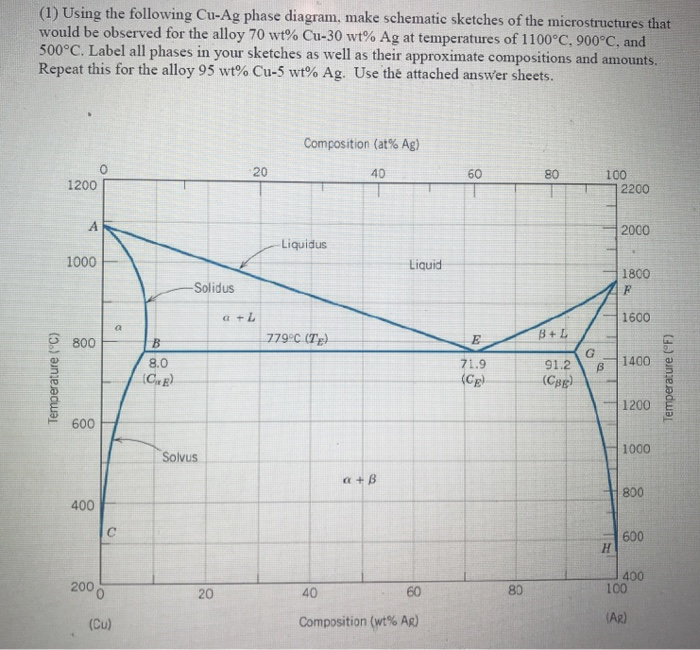
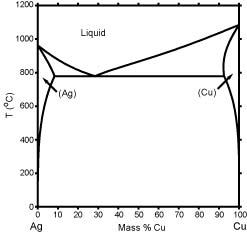
Ag-cu phase diagram. MSE 2090: Introduction to Materials Science Chapter 9, Phase Diagrams 23 Binary Eutectic Systems (II) Three single phase regions (α- solid solution of Ag in Cu matrix, β= solid solution of Cu in Ag matrix, L - liquid) Three two-phase regions (α+ L, β+L, α+β) Solvus line separates one solid solution from a mixture of solid solutions. Ag-45 wt% Cu composition at 900 °C. As may be noted, point C lies within the Liquid phase field. Therefore, only the liquid phase is present; its composition is 55 wt% Ag-45 wt% Cu. (d) The Mg-Pb phase diagram (Figure 9.20) is shown below; the point labeled “D” represents the 30 w t% Pb-70 wt% Mg composition at 425 °C. Ag Cu Phase Diagram – Metals Free Full precipitation hardening copper alloys electrical contacts besides the naturally hard copper materials precipitation hardening copper alloys play also an important role as carrier as the phase diagram Ag Cu Phase Diagram – Anchoring Di And Tri Metallic Nanoparticles An Amorphous PHASE DIAGRAMS PROBLEM SOLUTIONS 9.17 A 90 wt% Ag-10 wt% Cu alloy is heated to a temperature within the β + liquid phase region. If the composition of the liquid phase is 85 wt% Ag, determine: (a) The temperature of the alloy (b) The composition of the β phase (c) The mass fractions of both phases Solution
Download scientific diagram | Ag–Cu phase diagram. from publication: Brazing of copper to stainless steel with a low-silver-content brazing filler metal | The brazing of copper to stainless ... Thus, the phase compositions are as follows: Cα= 16 wt% Sn-84 wt% Pb Cβ= 97 wt% Sn-3 wt% Pb (c) The Ag-Cu phase diagram (Figure 9.7) is shown below; the point labeled “C” represents the 55 wt% Ag-45 wt% Cu composition at 900°C. As may be noted, point C lies within the Liquid phase field. Use the following silver-copper phase diagram for Problems 5-9. 5. What are the solubility limits of Ag in (Cu) and Cu in (Ag)? Recall that (Cu) and (Ag) ...4 pages 01.02.2007 · G. Leloup,“The Cu-Ag Phase Diagram as a Function of Pressure, ... D.G. Beck, S.M. Copley, and M. Bass,“The Microstructure of Metastable Phases in Ag-Cu Alloys Generated by Continuous Laser Melt Quenching, ”Metall. Trans. A, 12, 1687–1692 (1981). (Meta Phases, Crys Structure; Experimental) Article Google Scholar 81Bec2: D.G. Beck, S.M. Copley, and M. …
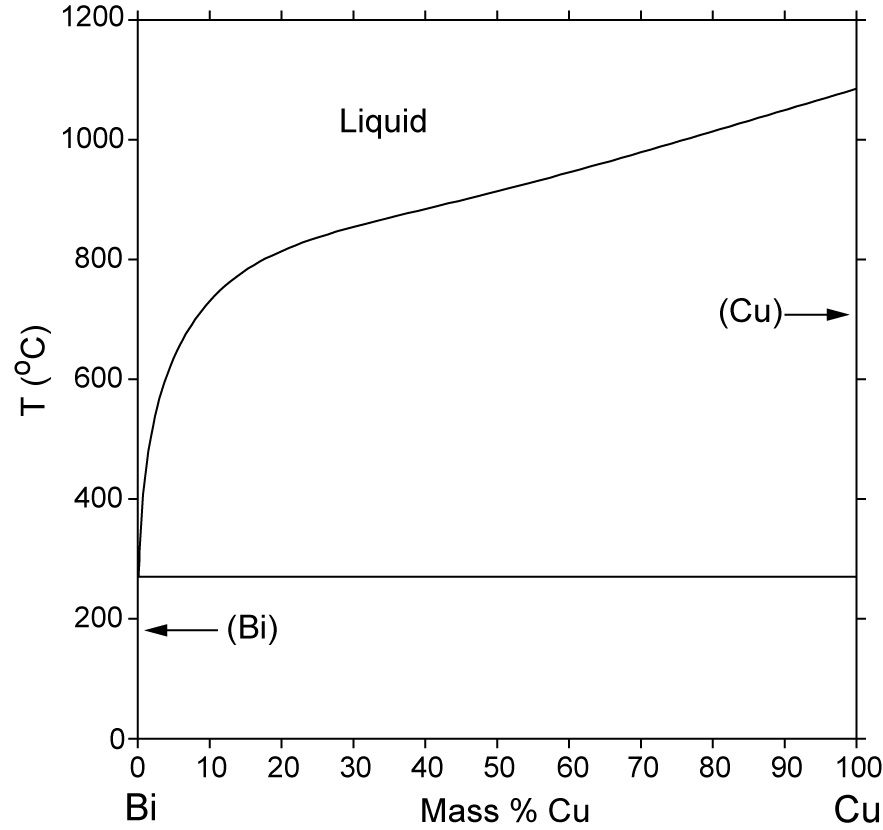
Mar 01, 2021 · The Ag–Cu phase diagram at nanoscale has been re-investigated via thermodynamic modeling, considering the surface effect on the chemical potential of pure substance and excess Gibbs free energy of mixtures. A self-consistent set of thermodynamic parameters has been obtained by considering the reported melting behavior of Ag, Cu, and Ag–Cu NPs. Ag-Cu System · Calculated Phase Diagram · Calculated Invariant Equilibria · Phases, Crystal Structures and Model Descriptions · Phase, Struktur- bericht. Symbol ... The phase diagram of the Ag-CuO x. system was experimentally constructed. An eutectic reaction CuO + Ag = L 1 (99at%Ag) at 1215K and a monotectic reaction CuO + L 1 (98at%Ag) = L 2 (34.5at%Ag) at 1242K were detected in air. TG experiments showed the weight loss at the monotectic temperature due to oxygen evolution. The ternary alloy system Ag-Cu-Si was investigated in detail for the first time. The phase diagram was analysed by means of light optical microscopy, powder x-ray diffraction, differential thermal analysis (DTA) and scanning electron microscopy in combination with energy dispersive x-ray spectroscopy.
The Ag-Cu phase diagram is given in the figure below. According to this phase diagram, what is: The melting temperature of pure Ag The melting temperature of pure Cu The maximum solubility of Cu in Ag@ 5009C The maximum solubility of Ag in Cu at 600°C The eutectic composition The eutectic temperature The phase rich in Cu The phase rich in Ag Composition (at% Ag) 100 2200 20 1200 1000 Liquid 1800 1600 a +L 800 1400 1200 600 1000 200 100 20 60 Composition (wt% Ag)
Sn-rich alloys in the Sn-Ag-Cu system are being studied for their potential as Pb-free solders. Thus, the location of the ternary eutectic involving L, (Sn), Ag3Sn and Cu6Sn5 phases is of critical interest. Phase diagram data in the Sn-rich corner of the Sn-Ag-Cu system are measured. The ternary eutectic is confirmed to be at a composition of 3.5
L + Cu 3 Sn -> Ag 3 Sn + Cu 6 Sn 5. 356.2 o C: Liquid: 19.26: 5.86: 74.88: Cu 3 Sn: 0: 61.63: 38.37: Ag 3 Sn: 73.17: 0: 26.83: Cu 6 Sn 5: 0: 39.07: 60.93: L -> Ag 3 Sn + Cu 6 Sn 5 + (Sn) 215.9 o C: Liquid: 3.73: 0.85: 95.42: Ag 3 Sn: 73.17: 0: 26.83: Cu 6 Sn 5: 0: 39.07: 60.93 (Sn) 0.07: 0: 99.93: L + gamma + Cu 3 Sn 677.0 o C. Univariant maximum: Liquid: 7.51: 48.64: 43.83: gamma: 0: 61.63: 38.37: Cu 3 Sn: 0: 61.63: 38.37















0 Response to "40 ag-cu phase diagram"
Post a Comment