37 given the potential energy diagram for a reaction
Transcribed Image Textfrom this Question. H3.25-Level 1 Given the potential energy diagram, calculate the activation energy for the catalyzed reaction (kJ). Without catalyst 1801 160 140 120 100 80 60 40 With catalyst 0. 20 Reaction coordinate. Potential Energy Diagrams A potential energy diagram plots the change in potential energy that occurs during a chemical reaction. This first video takes you through all the basic parts of the PE diagram. Potential Energy Diagram Basics (PE Diagrams) Watch later Watch on
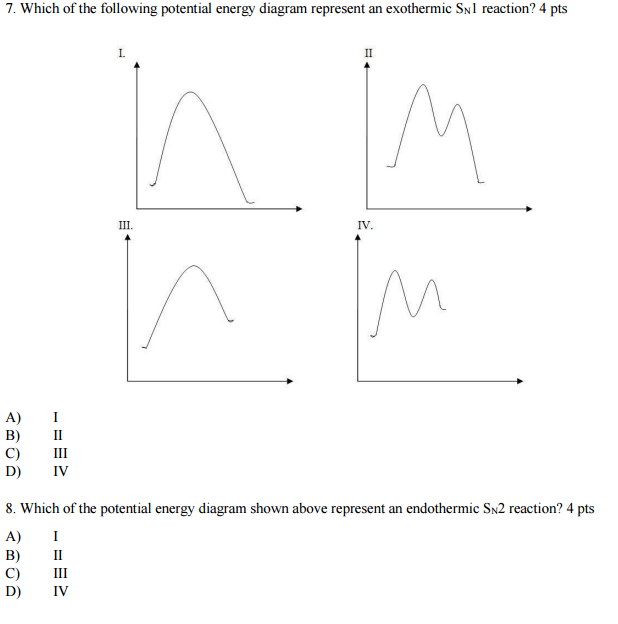
11. The following potential energy diagram refers to a very slow one-step reaction of ozone (O3) and oxygen atoms in the upper atmosphere. 150 On the axis below, draw a potential energy diagram which could represent the catalyzed mechanism for the reaction: Step 1: O3 + NO à NO2 + O2 (slow) Step 2: NO2 + O à NO + O2 (fast) 0 50 100 150
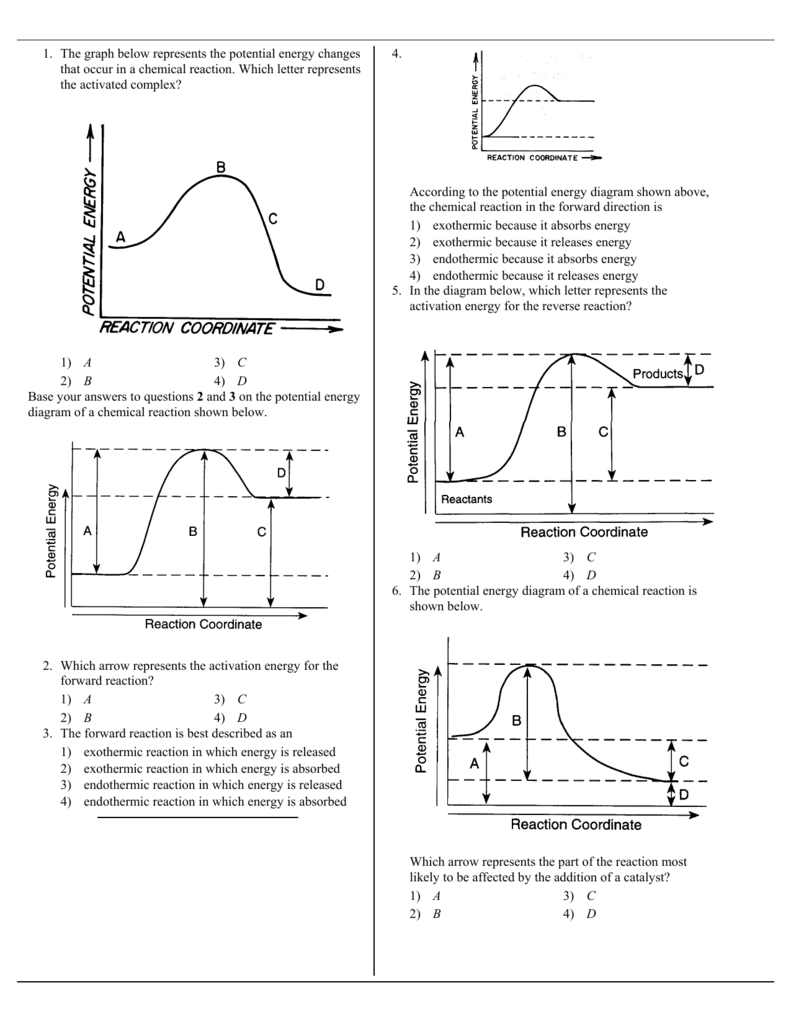
Given the potential energy diagram for a reaction
We have to draw a potential energy diagram for the given reaction: C 3 H 8(g) + 5 O 2(g) → 3 CO 2(g) + 4 H 2 O (g) + 2219.9 kJ. This reaction is the combustion of propane (C 3 H 8). Combustion is a reaction where a compound burns in oxygen to produce carbon dioxide (CO 2) and water (H 2 O). The potential energy diagram for the above reaction is as shown below This reaction follows the substitution nucleophilic bimolecular mechanism.The given reaction is said to be an exothermic ... Shows how a potential energy diagram can be used to determine activation energy and enthalpy change (Delta H) for forward and reverse reactions. Shows how a ...
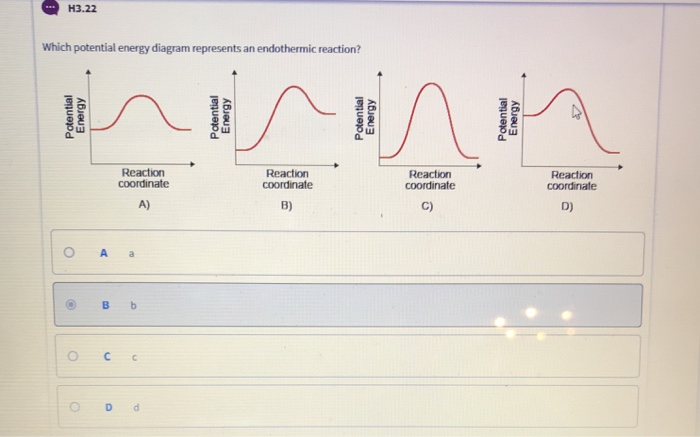
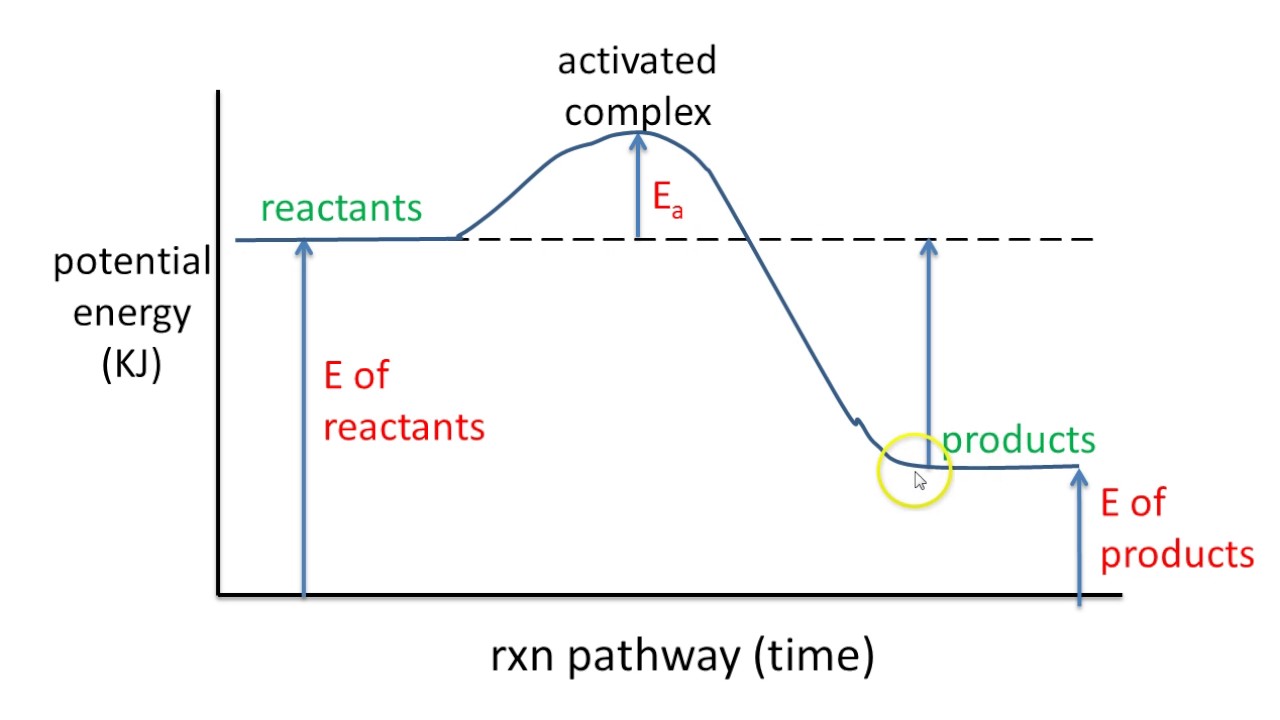
Given the potential energy diagram for a reaction. Given the potential energy diagram for a reaction: A) Identify the segment that describes the activation energy of the reaction. Section 2 B) Draw a dotted line to show the new activation energy when a catalyst is added C) Explain the effect catalysts have on reactions it increases the rate of a chemical reaction 4. Thermodynamics Review 27. Given the potential energy diagram: With reference to energy, the reaction A + B → AB can best be described as 1) endothermic, having a +ΔH 2) endothermic, having a -ΔH 3) exothermic, having a +ΔH 4) exothermic, having a -ΔH 28. Apr 09, 2018 · Apr 9, 2018. Since heat is released for. C3H8(g) + 5O2(g) → 3CO2(g) +4H2O(g) + 2219.9 kJ, we say that ΔH ∘ C = − 2219.9 kJ/mol propane. We approximate that this is the change in potential energy for the reactants going to the products. The above is for an endothermic reaction. A certain feature of combustion reactions suggests that you should draw the diagram DIFFERENTLY from what you see above. The energy changes that occur during a chemical reaction can be shown in a diagram called a potential energy diagram, or sometimes called a reaction progress curve. A potential energy diagram shows the change in potential energy of a system as reactants are converted into products. The figure below shows basic potential energy diagrams for an endothermic (A) and an exothermic (B) reaction.
AIIMS 2003: The potential energy diagram for a reaction R → P is given in the figure. Δ H ° of the reaction corresponds to the energy (A) a (B) Given the potential energy diagram for a chemical reaction: Which statement correctly describes the energy changes that occur in the forward reaction? A. The activation energy is 10. kJ and the reaction is C. The activation energy is 50. kJ and the reaction is endothermic. endothermic. B. HELP ASAP I WILL GIVE BRAINLIST The potential energy diagram for a reaction starts at 380 kJ and ends at 100 k… Get the answers you need, now! asked May 23, 2019 in Chemistry by AtulRastogi (91.3k points) closed Nov 20 by AtulRastogi The potential energy diagram for a reaction R → P R → P is given below. ΔH ɵ Δ H ɵ of the reaction corresponds to the energy A. x x B. y y C. z z D. (x + y) ( x + y) class-12 chemical-kinetics 1 Answer 0 votes ← Prev Question Next Question →
The reaction NO2 (g) + CO (g) → NO (g) + CO2 (g) has ΔH°overall = -226 kJ/mol. A proposed reaction mechanism is shown. Choose the statement (s) that accurately describe the reaction energy diagram for the above reaction. -There will be three peaks. -The Ea of the first step will be larger than the second or third step. Given the potential energy diagram for a reversib I I Given the reaction: + energy Which diagram best represents the potential en gy changes for this reaction? chemical reaction: Reaction Coordinate Each interval on the axis labeled "Potential Energy (kJ/moI)" represents 10. kilojoules per mole. Wha is the activation energy of the forward reaction? A potential energy diagram represents the change in potential energy of a system as reactants are changed into products. Complete step by step answer: We have to know that the Arrhenius equation is helpful in calculating the rate of reaction and plays an important portion in chemical kinetics. We can write Arrhenius equation as, 14.Given the potential energy diagram for a reaction: ... 36.A potential energy diagram for a chemical reaction is shown below. On this diagram, draw a curve to show how the potential energy diagram will change when a catalyst is added to the reaction. Name Unit 10 Practice Test
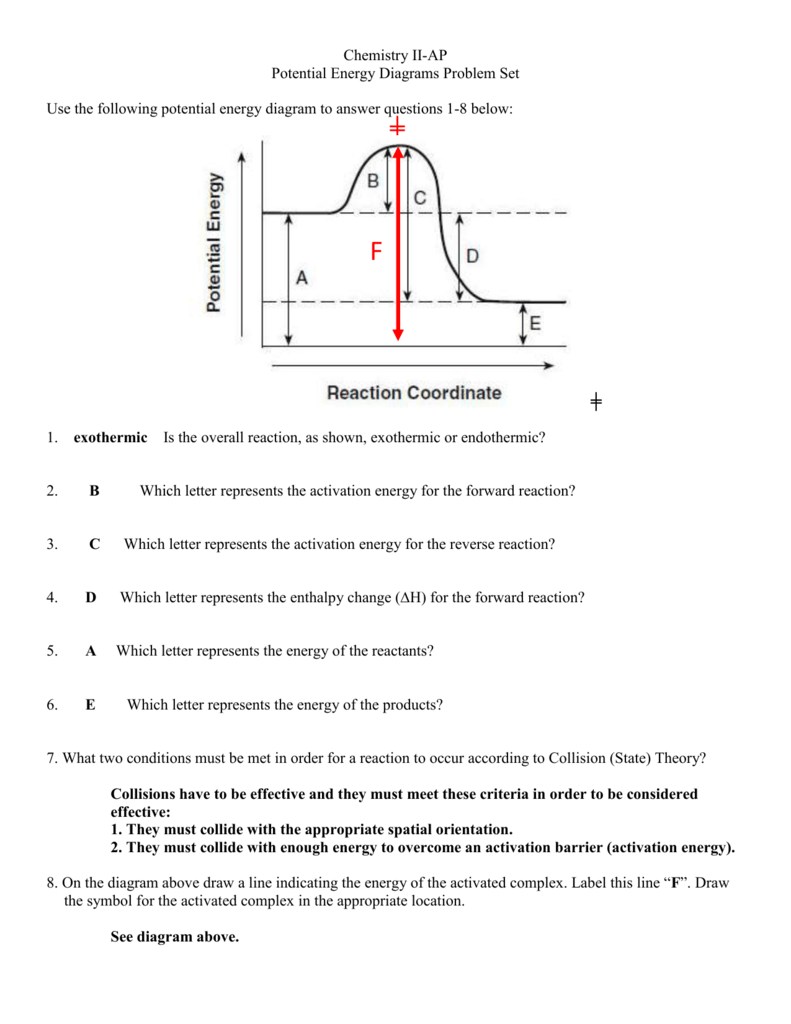
A→ activation energy, C → H ∘. Activation energy is defined as the least possible amount of energy (minimum) which is required to start a reaction or the amount of energy available in a chemical system for a reaction to take place →A. C → H o is the difference of the forward and backward activation energies. Hence, the correct answer is option A.
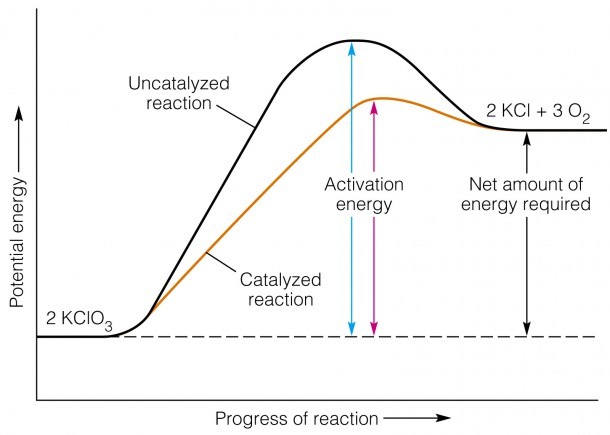
The potential energy diagram below represents a reaction. PE 40 Potential Energy Diagram pathway without with catalyst Course Ca culate the heat o reaction. What effect does a catalyst have in a ... potential energy that occur during the given reaction. Given the reaction: A + B --> C a) Does the diagram illustrate an exothermic or an ...
The potential energy diagram shown below represents the reaction a + b + 50 kJ c + Reaction Coordinate Given the potential energy diagram: Reaction Coordinate Does this potential energy diagram represent an exothermic or an endothermic reaction? [Explain why.] Which lettered interval represents the potential energy of the actants of the
Reaction Rates and Potential Energy Diagrams 1. Chemical reactions occur when reactants collide. For what reasons may a collision fail to produce a chemical reaction? Not enough energy; improper angle. 2. If every collision between reactants leads to a reaction, what determines the rate at which

All wind turbines side by side producing pure electricity without destroying our beloved planet Earth.
Solution for 1. Given the potential energy diagram below, indicate what each interval labelled by the letter represents. B C A Reaction Coordinate a) Interval…
On the diagram below, draw a potential energy diagram for this reaction. 18. Base your answer(s) to the following question(s) on the reaction represented by the balanced equation below. 2H2(g)+O2(g) !2H2O(')+571:6kJ On the axes below, draw a potential energy diagram for the reaction represented by this equation. 19. Given the reaction at ...
r based upon the given potential energy diagram and indicate if the reaction endothermic or exothermic. What Is Given? You have a potential energy diagram. Plan Your Strategy Act on Your Strategy Refer to the scale on the y-axis and determine the difference in energy between the reactants and the transition state, E a(fwd); the difference in energy
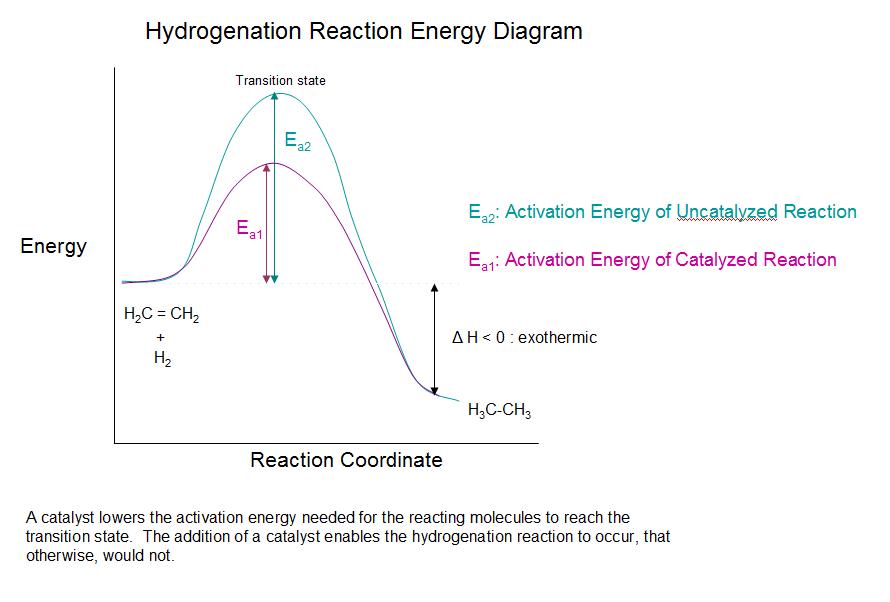
Figure Potential energy diagram of catalyzed vs uncatalyzed reaction pathway. Potential energy. Energy Diagrams for Catalyzed and Uncatalyzed Reactions. Page 2. Page 3. Page 4. Enzymatic Catalysis of a Reaction between Two Substrates. Page 5. Enthalpy profile for an non-catalysed reaction, last page a typical, non- catalysed reaction can be ...
The potential energy diagram for a reaction R → P is given below. ... Consider the potential energy diagram given below In accordance with Hammond's postulate, exothermic reactions tend to have _____. Hard. View solution > The threshold energy of the reaction is: Medium. View solution >
Problem: The graph shows the potential energy diagram for a given chemical reaction. What is the heat of reaction for the forward reaction? FREE Expert Solution 83% (103 ratings) Sign up for free to view this solution Sign up for free. 562,230. students enrolled. 97%. improved grades ...
15. The potential energ diagram below represents a reaction. Reaction Coordinate Which arrow represents the activation energy of the forward reaction? 16. Given the potential energy diagram of a chemical reaction: Reaction Coordinate Which arrow represents the potential energ of the reactants? 17. Given the reaction: S(s) + 02(g) -+ S02(g) + energy
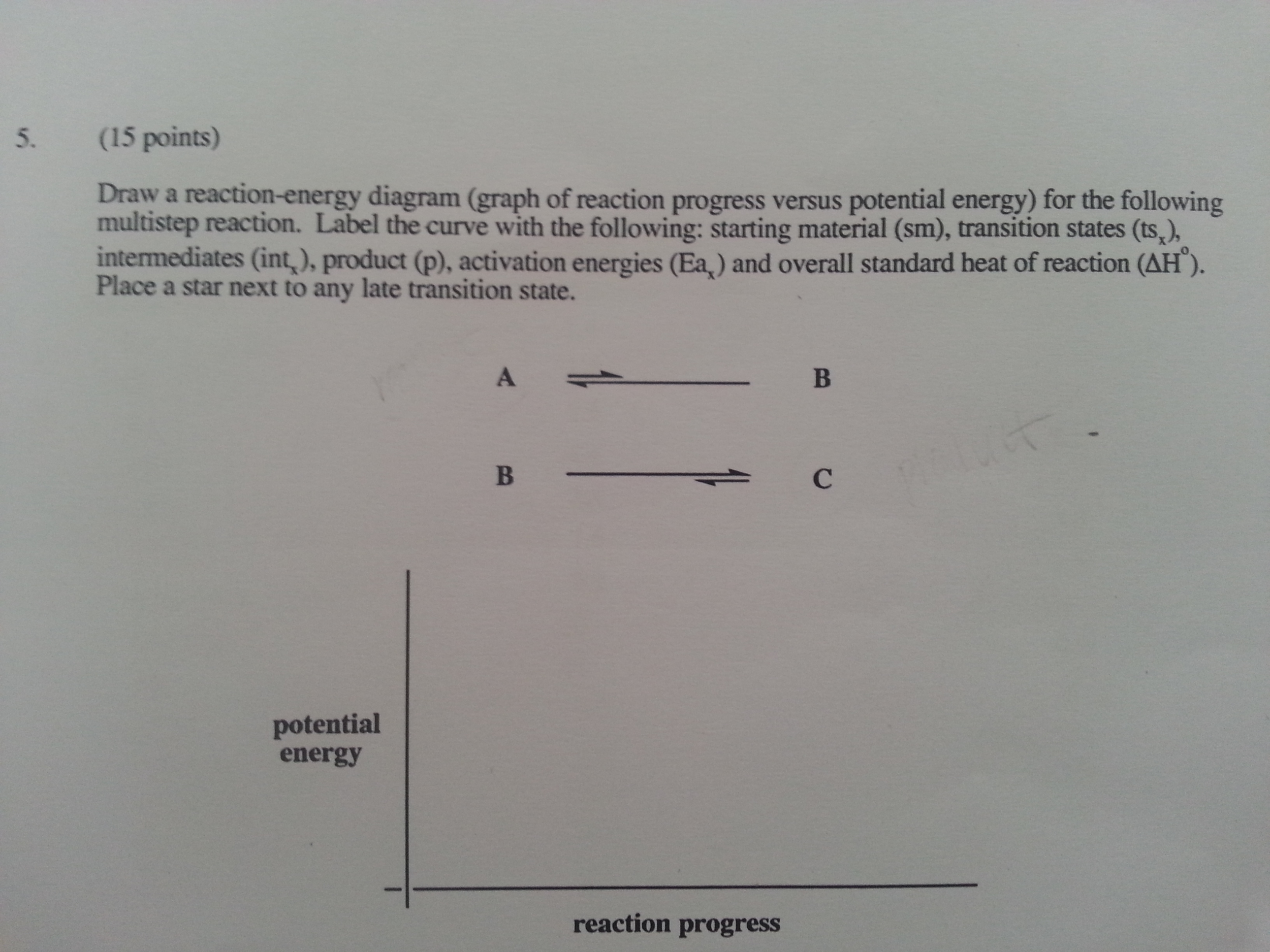
Transcribed image text: Given the following potential energy diagram for a 3 step reaction, label or identify which arrow shows these categories for both the forward and reverse reactions (do forward in one color and reverse in a different color): a.) Reactant(s) b.) Product(s) c.) Intermediate(s) d.) Rate determining step e.) ist activation energy f.) 2nd activation energy g.)
Shows how a potential energy diagram can be used to determine activation energy and enthalpy change (Delta H) for forward and reverse reactions. Shows how a ...
The potential energy diagram for the above reaction is as shown below This reaction follows the substitution nucleophilic bimolecular mechanism.The given reaction is said to be an exothermic ...
We have to draw a potential energy diagram for the given reaction: C 3 H 8(g) + 5 O 2(g) → 3 CO 2(g) + 4 H 2 O (g) + 2219.9 kJ. This reaction is the combustion of propane (C 3 H 8). Combustion is a reaction where a compound burns in oxygen to produce carbon dioxide (CO 2) and water (H 2 O).

(Fang Ruida) Davis. K Coronavirus pneumonia biomarkers and treatment(方瑞达) Davis。K å† çŠ¶åž‹ç—…æ¯’æ€§è‚ºç‚Žç”Ÿç‰©æ ‡å¿—ç‰©åŠæ²»ç–—防治

SAKURAI - THE MIND-BLOWING CAPTURE OF AN ELECTRON OBITAL --- THE NEW HIGH-ENERGY IMAGES AT CERN OF A HELIUM ANTIMATTER / MATTER NUCLEON IN REAL-TIME AND CAPTURED AT THE SPEED OF LIGHT.





















0 Response to "37 given the potential energy diagram for a reaction"
Post a Comment