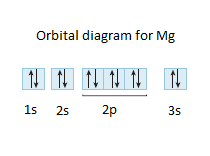
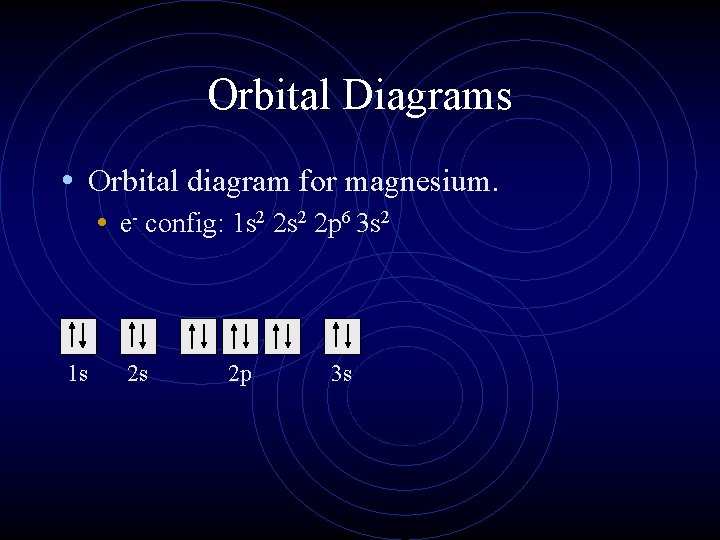
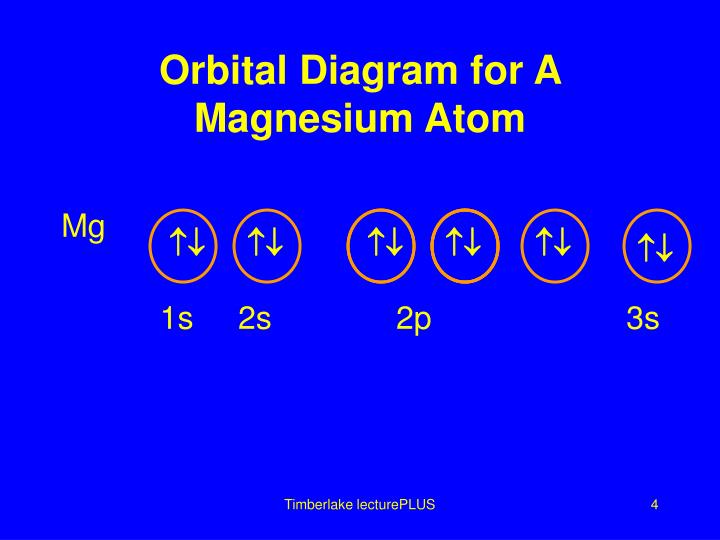
40 orbital diagram for magnesium
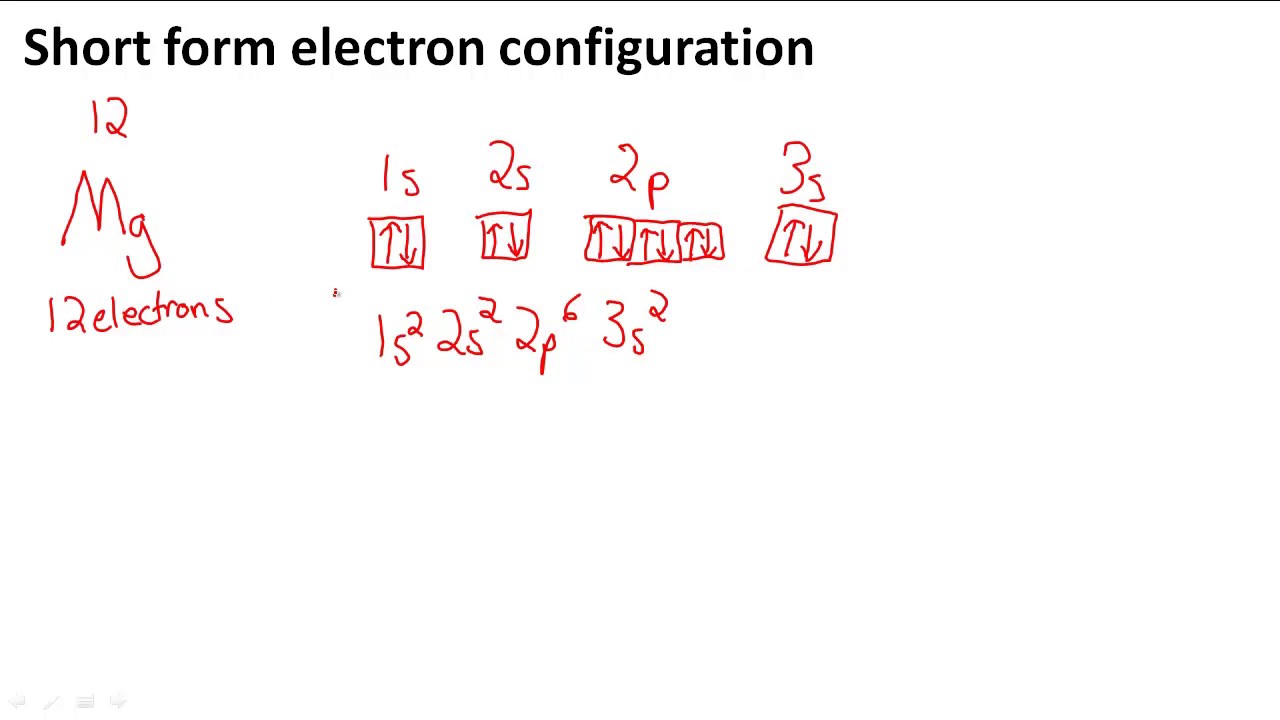
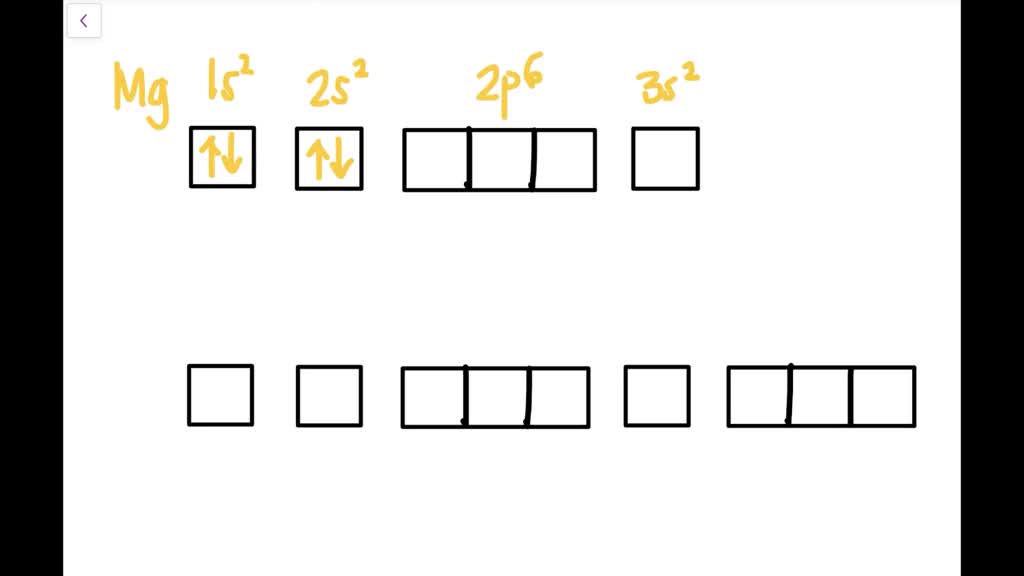
The nex six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the remaining two electrons in the 3s. Therefore the Magnesium electron configuration will be 1s22s22p63s2. What is the dot diagram of magnesium oxide? Magnesium is able to bond with one oxygen atom.
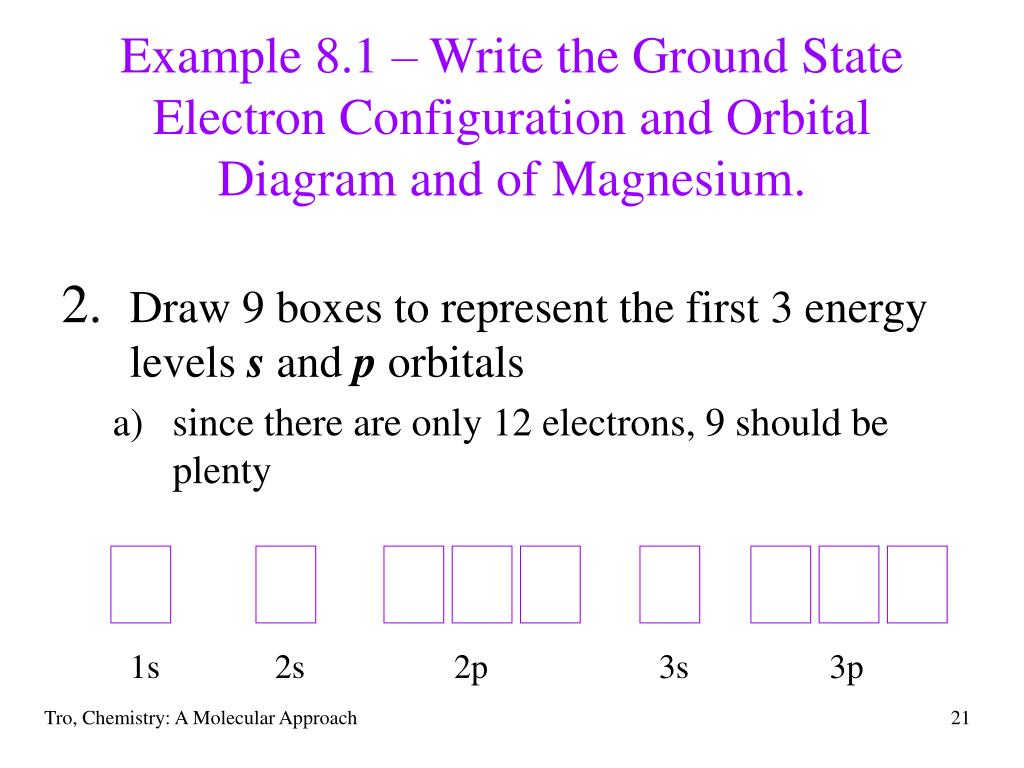
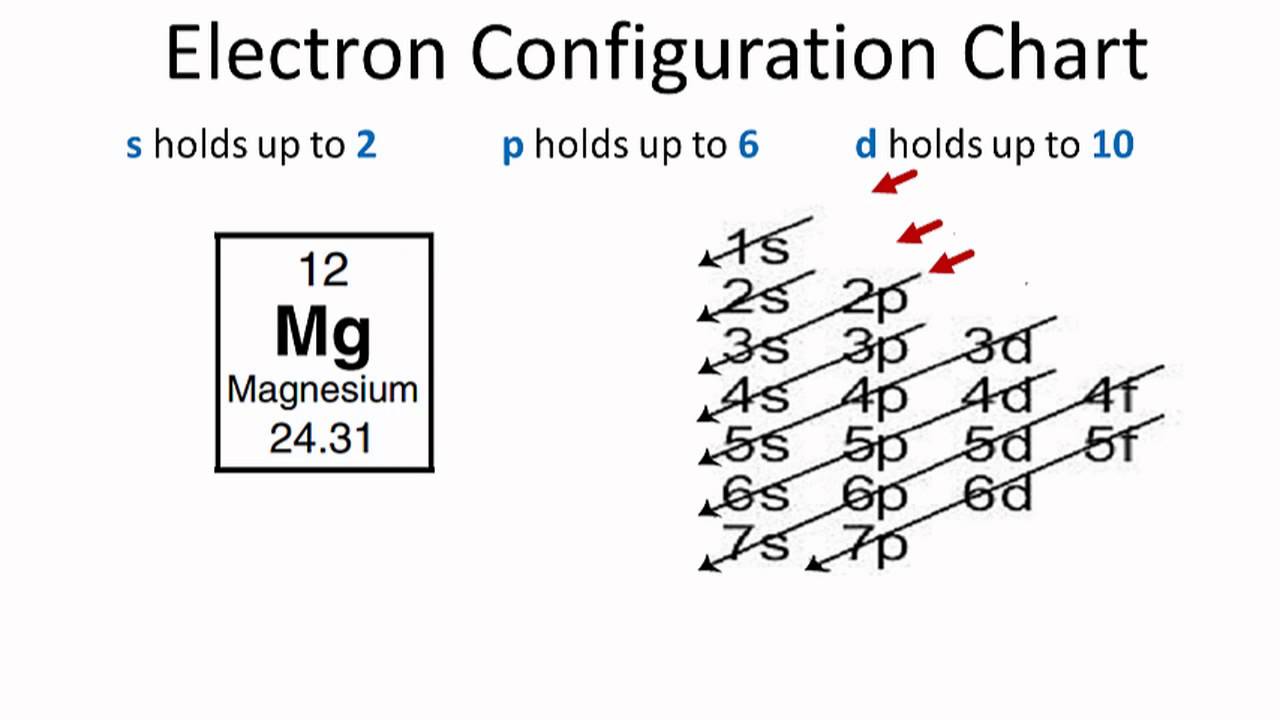
First ionization energy is the energy required to remove one electron from the gaseous atom. 1s2222p63s2 The Aufbau diagram can help you determine the correct order in which the electrons will fill the orbitals in an atom. The atomic number of Magnesium is 12. Magnesium is in the third row and second column on the periodic table.
Magnesium cation (Mg2+) is, as stated above, is a cationic /less stable derivative of Mg formed after It gives up two of its valency or outer electrons from its electron shell or orbital. Electronic Configuration of Mg2+ tells us how many electrons are arranged in each shell of the Mg2+ atom and its shape. Mg2+ has an electronic configuration ...

Orbital diagram for magnesium
Spdf notation and orbital box diagram 0 pages · 0 · 0 b · 0 downloads orbital diagrams and electron configuration. In magnesium oxide, oxygen is in a … 3 electron configuration • 2. To save room, the configurations are in noble gas shorthand.this means part of the electron configuration has been replaced with the element symbol of the ...
This is a diagram of the Periodic Table. As we can see, Mg belongs to group 2 and has an atomic number of 12 whereas Cl belongs to group 17 and has an atomic number of 17. Mg has 2 valence electrons whereas Cl has 7 valence electrons. The total number of valence electrons in a molecule of magnesium chloride = 2*1 + 7*2 = 16.
Short answer: Mg is ; its Noble gas shorthand is . There are two rules to keep in mind, whenever you are trying to figure out the electron configuration of any ...1 answer · 3 votes: This just shows energy levels so let's take this a step further. Atomic Electron Configurations ...
Orbital diagram for magnesium.
Magnesium (Mg) has an atomic mass of 12. Find out about its chemical and physical ... Electron Configuration, [Ne] 3s2. 1s2 2s2 2p6 3s2. Orbital Diagram.
How do you write the electron configuration for magnesium?, The electron configuration for magnesium is 1s 2 2s 2 2p 6 3s 2.. Furthermore, What is the electron configuration of magnesium atomic number 12?, Magnesium, atomic number 12, has the electron configuration [Ne]3s^2. Finally, What is the electron configuration for an Mg2+ ion?, Therefore the Magnesium electron configuration will be 1s ...
In the case of magnesium oxide (MgO) both the participating atoms are in the form of ions that are not directional, so they cannot have molecular geometry, hybridization, and molecular orbital diagram. The non-compliance to covalent properties and ionic bonding can further be explained with the help of the electronegativity concept.
Carbon Electron Dot Diagram. If we talked about the electronic configuration of the element then, carbon is an element whose electronic configuration is given as 1s22s22p2. Now the main thing here is what electronic configuration actually means, so the solution/ answer is that in simple words, by knowing the electronic configuration of any ...
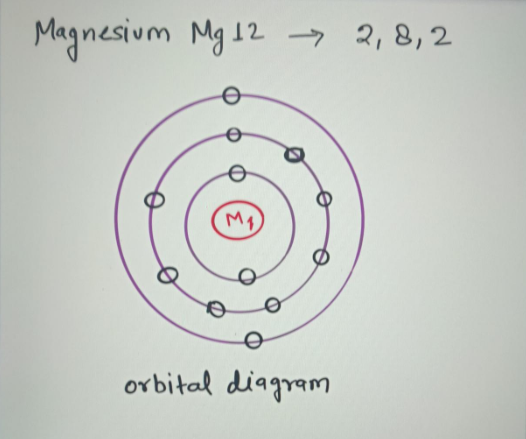
Magnesium Mg has an atomic number of 12. Magnesium Electron Configuration Mg With Orbital Diagram . Chemistry 18112019 0831 coxtinam16. Ground state electron configuration of magnesium atom. The electron configuration for ground state would then be. Magnesium atoms have 12 electrons and the shell structure is 282.
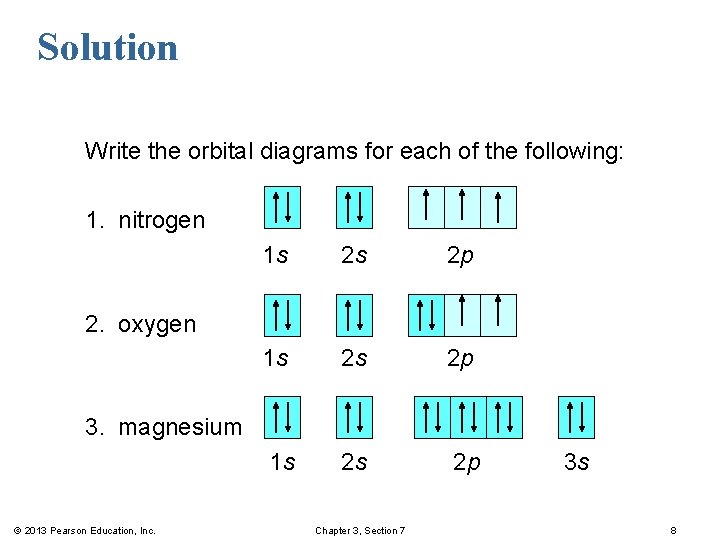
What is the Orbital Diagram For Nitrogen? When we talk about the orbital diagram, we first need to understand what exactly it means. Therefore, during exams, the student can expect questions related to this topic so it is important that the students must go through it.
1 answerThe orbital notation for magnesium is: Mg_notation. Magnesium is in the third row and second column of the Periodic Table. This location on the Periodic...
This Is Important because Valence Electrons add To The unique Chemistry Of every Atom. This Is essential When describing An Electron configuration In terms of The orbital Diagrams. What Is The soil State Electron configurations Of Magnesium? Magnesium Atoms have actually 12 Electrons and also The covering Structure Is 2.8.2.
Electron Configuration Chart of All Elements (Full Chart) June 10, 2021 March 7, 2021 by Admin. Electron configuration chart of all Elements is mentioned in the table below. The Shorthand electron configuration (or Noble gas configuration) as well as Full electron configuration is also mentioned in the table. Atomic no.
In writing the electron configuration for Magnesium the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons ...Oct 24, 2016 · Uploaded by Wayne Breslyn
Magnesium Electron Configuration (Mg) with Orbital Diagram. Magnesium Electron Configuration: Mg is a chemical element that has the symbol Mg. The atomic number of Magnesium is 12. It is a grey shiny solid that bears a close physical resemblance to five other elements of the second column and group 2 (alkaline earth metals) of the periodic table.
Orbital Diagram 1s ↿⇂ 2s ↿⇂ 2p ↿⇂ ↿⇂ ↿⇂ 3s ↿⇂ 3p ↿⇂ ↿⇂ ↿⇂ 3d ↿⇂ ↿⇂ ↿⇂ ↿⇂ ↿⇂ 4s ↿⇂ 4p ↿⇂ ↿ ↿ 4d 4f: ... Is it hard or easy to remove an electron from magnesium? The electron being removed from a Mg atom is a 3 s electron, which is only shielded by the inner core electrons. ...
Electronic configuration of the Magnesium atom. Valence electrons. Orbital diagram.
Magnesium Cation Mg2+. Magnesium cation (Mg2+) is, as stated above, is a cationic /less secure derivative of Mg developed after It gives up two of that valency or external electrons from its electron shell or orbital.Electronic configuration of Mg2+ tells us how many electrons are arranged in each covering of the Mg2+ atom and also its shape.
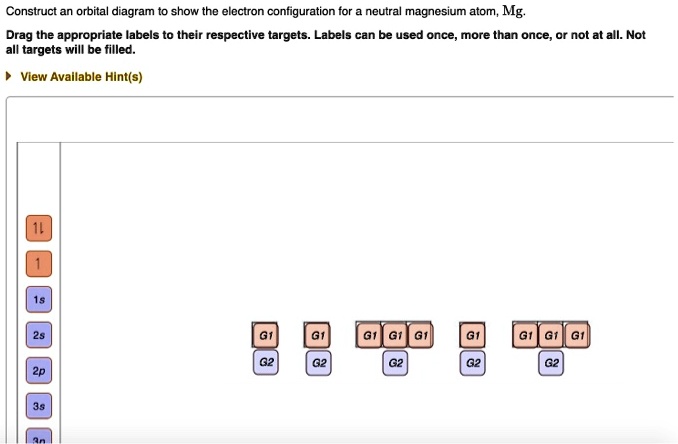
For that, we first need to determine the electron configuration of Mg. Recall that for a neutral element, Atomic number = # of protons = # of electrons. The ...Aug 12, 20191 answer · Top answer: We’re being asked to construct the orbital diagram for Mg. For that, we first need to determine the electron configuration of Mg.Recall that for a ...
Answer (1 of 2): I'm just going to answer your question…Please understand that electron configuration is more complex than what is being presented to you. Here is how this all works out, and I'm going to use Mg (the element you presented) as my example. Magnesium (Mg) is the 12th element on the...
The electron configuration for magnesium is 1s2 2s2 2p6 3s2. The K shell contains a 1s subshell hence it can carry 2 electrons the L shell has 2s and 2p and can carry 8 electrons. Ne 3s 2 Ne 3s 2. The p orbital can hold up to six electrons. The N shell containing 4s 4d 4p and 4f can carry 32 electrons.
The degree of freedom at triple point in unary diagram for water _____. (a) 0 (b) 1 (c) 2 (d) 3 . 4. Following is wrong about a phase diagram. ... .chlorides and sulfates of calcium and magnesium . d).phosphates of sodium and potassium ... 53.On the basis of molecular orbital theory, select the most appropriate option. a) The bond order of O2 ...
The nex six electrons will go in the 2p orbital. The orbital notation for magnesium is: Magnesium is in the third row and second column of the Periodic Table. what is the orbital notation of magnesium and silicon? Since 1s can only hold two electrons the next 2 electrons for magnesium go in the 2s orbital.
Orbital diagram of Lithium (Li) 4: Orbital diagram of Beryllium (Be) 5: Orbital diagram of Boron (B) 6: Orbital diagram of Carbon (C) 7: Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine (F) 10: Orbital diagram of Neon (Ne) 11: Orbital diagram of Sodium (Na) 12: Orbital diagram of Magnesium (Mg) 13 ...
Orbital Diagram: The subatomic particle that occupies most of the volume of an atom is known as the electron. The region of space wherein electrons ... That is, magnesium is a cation element. Magnesium donates the electron of the last shell to form bonds and turns into magnesium ions. Mg - 2e - → Mg +2.
Magnesium has an electronic configuration of 282. Magnesium bromide electron configuration. 47 1959 x 2 of 4 Describe the electron transfers that occur in the formation of magnesium bromide from elemental magnesium and elemental bromine. Hence it has a charge of 2 Mg2. The nex six electrons will go in the 2p orbital.
The lewis dot structure for magnesium is an mg with 2 dots which stand for its two valence electrons. What is an bond electron transfer how electron dot diagram for s awesome sulfur atom flow block orbital diagram for magnesium awesome lewis electron dot diagram s. Magnesium reacts with sulfur to produce sulfide a in. That is, magnesium is a ...
Konfigurasi Elektron, Diagram Orbital, Contoh Soal, dan Pembahasannya Gurubagi.com. Atom terdiri atas inti dan elektron yang beredar mengitarinya menurut lintasannya masing-masing. Untuk mengetahui bagaimana lintasan elektron tersebut, maka dapat kita melihat penyebaran elektron dalam kulit-kulit elektron melalui konfigurasi elektron.
Answer: (1) Covalent bond: The chemical bond formed due to mutual sharing of electrons between the given pairs of atoms of non-metallic elements.In the bond formed by a shared pair of electrons, each bonding atom contributing one electron to the pair. Depending on number of electron pairs shared bond is single [-], double [=], or triple [ = ] covalent.
36 orbital diagram for magnesium ion The electron configurat ion of a neutral magnesium atom is: 1s2 2s2 2p6 3s2 or in shorthand [Ne] 3s2. Magnesium has 2 valence (outer...
Electron Configuration and Orbital Diagram for Magnesium. Orbital Diagrams Chemistry Tutorial Key Concep… Written By Zukowski Safters Tuesday, November 23, 2021 Add Comment Edit. Older Posts Home. Subscribe to: Posts (Atom) Popular Posts. ...
Magnesium is element #12. To draw the Lewis electron dot diagram we picture in our minds the symbol for Mg in a box with all of its core electrons (i.e., 1s 2 2s 2 2p 6). Then we place the valence electrons around the sides of the box with each side representing an orbital in the outermost energy level.

















![6] (a) Write an orbital diagram for the ground state of Magnesium ...](https://img.homeworklib.com/images/b7f46805-3b3a-4cf6-8603-c907004a6c53.png?x-oss-process=image/resize,w_560)


0 Response to "40 orbital diagram for magnesium"
Post a Comment