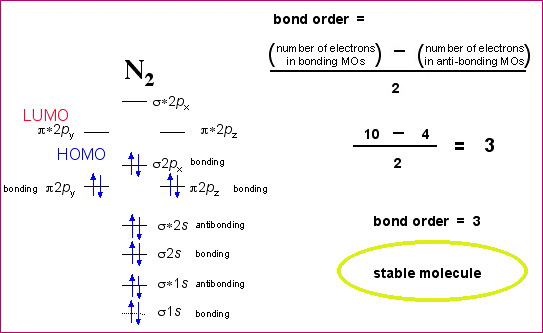
39 how to calculate bond order from mo diagram
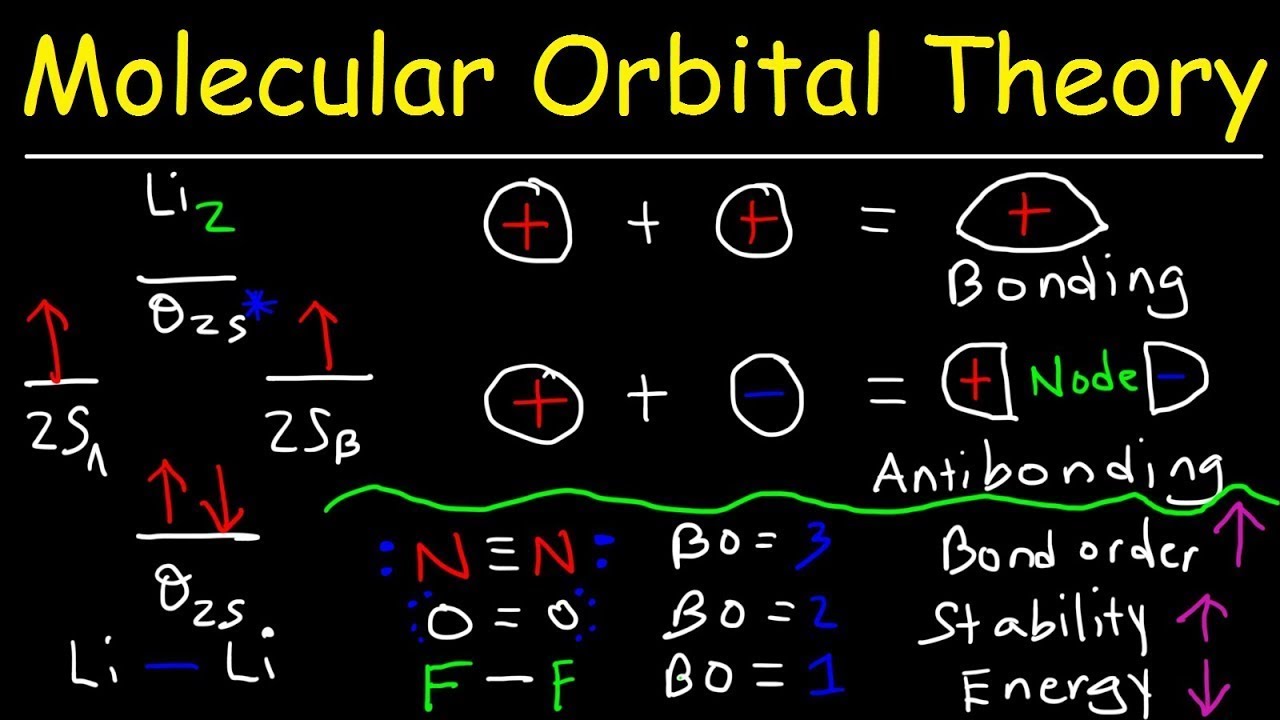
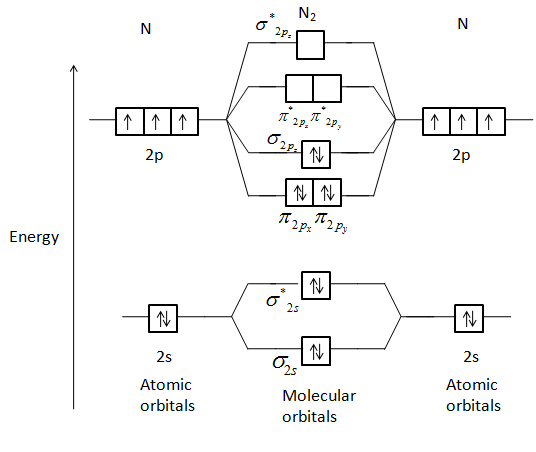
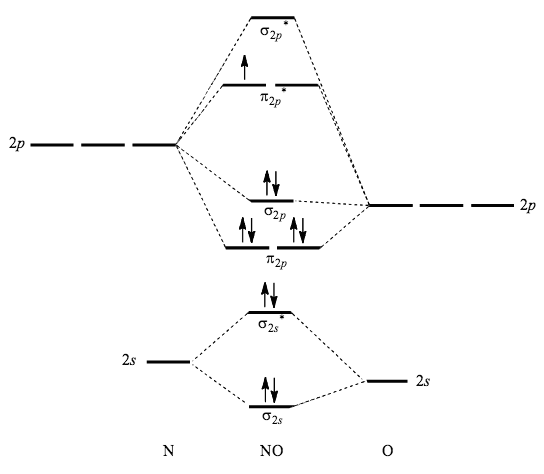
The molecular orbital diagram for C 2 molecule is :. The electronic configuration of C 2 is K K (σ2s) 2 (σ * 2s) 2 n (2px) 2 n (2py) 2. The C 2 molecule is diamagnetic because all electrons are paired there are no unpaired electrons. Molecular orbital diagram for c2 2-. The bond order of B2, C2, and N2 are 1, 2, and 3, respectively.
Molecular Orbital Diagrams, Bond Order, and Number of Unpaired Electrons Draw the molecular orbital diagram for the oxygen molecule, O 2. From this diagram, calculate the bond order for O 2. How does this diagram account for the paramagnetism of O 2? Solution. We draw a molecular orbital energy diagram similar to that shown in Figure 11.
c. 1200, ordren, "give order to, to arrange in a row or rank," from order (n.). Sense of "set or keep in order" is from c. 1500. Meaning "to give commands for or to, instruct authoritatively" is from 1540s; that of "command to be made, done, or issued" is from 1763. Related: Ordered; ordering.
How to calculate bond order from mo diagram
Learn how to apply molecular orbital theory to determine the shapes of bonded orbitals, recognize molecular orbital diagrams, calculate bond order, and determine relative bond strength. Updated ...
Old English hu "how," from Proto-Germanic *hwo (source also of Old Saxon hwo, Old Frisian, Middle Dutch hu, Dutch hoe, German wie, Gothic hvaiwa "how"), an adverbial form from PIE root *kwo-, stem of relative and interrogative pronouns. Practically a doublet of why, differentiated in form and use. How come? for "why?" is recorded from 1848 [Bartlett]. Emphatic phrase and how! is recorded from 1865. The formulation was common in book and article titles ("The National Debt, and How to Pay It"), but Pennsylvania writer Bayard Taylor, in whom it is first recorded, seems to have regarded it as a German or German-American expression.
From this electronic configuration, we can see that there are a total of ten bonding molecular orbitals and eight antibonding molecular orbitals. Here, N a =10, N b =8 now put it into the formula of bond order. So, according to the formula of bond order: B.O. = Na-Nb/2 = [10-8]/2 = 1 which implies that f2 is combined with a single bond.
How to calculate bond order from mo diagram.
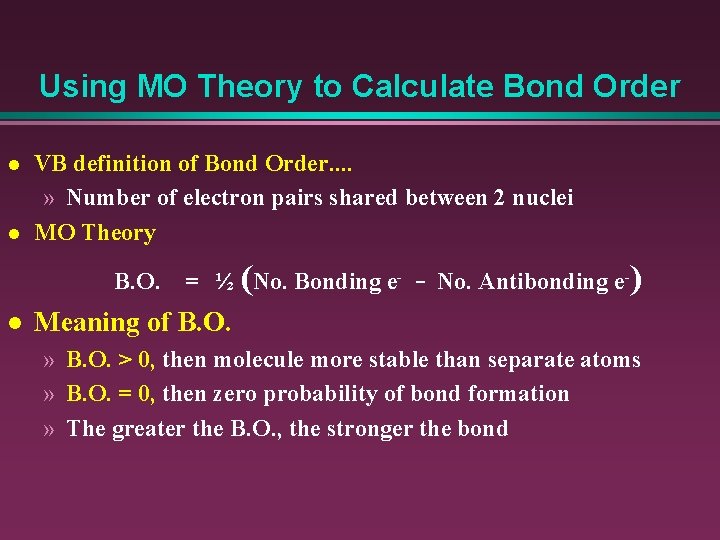
How do I calculate bond order? Bond order = [ (Bonding molecules' number of electrons) - (Antibonding molecules' number of electrons)]/2. Why is the bond order of CO 3? In molecular orbital theory, we define bond order as half of the difference between the number of bonding and antibonding electrons.
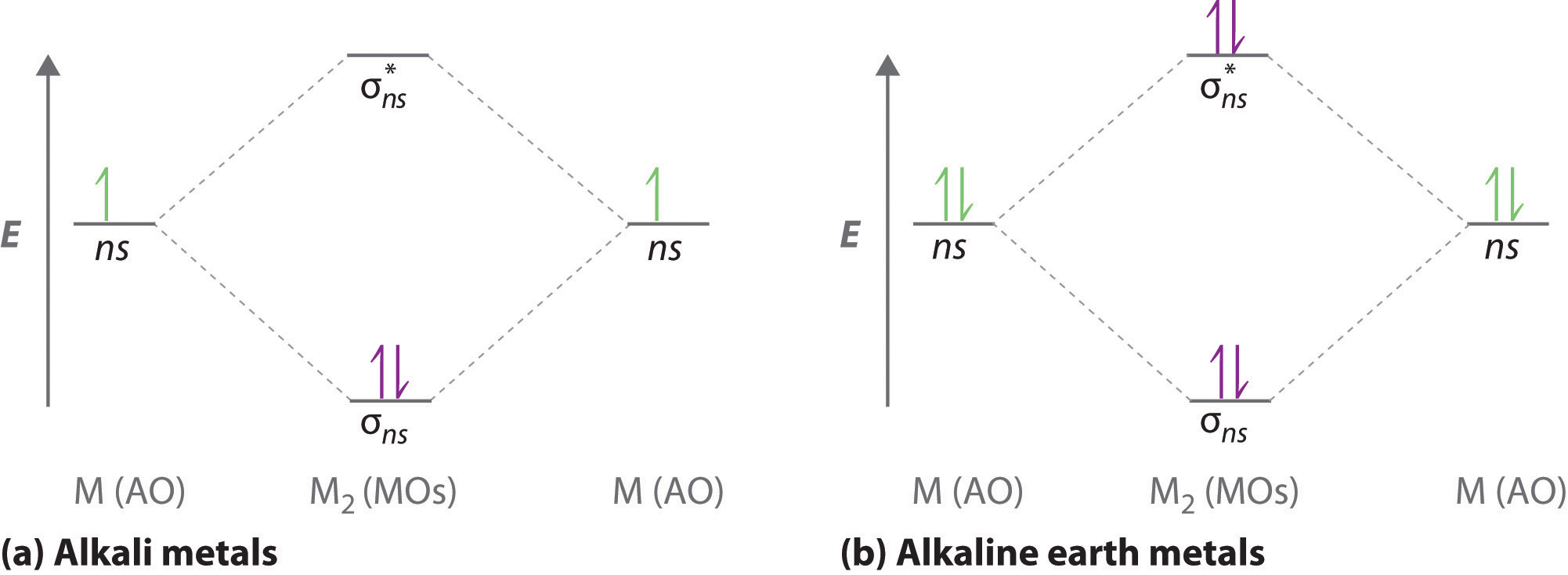
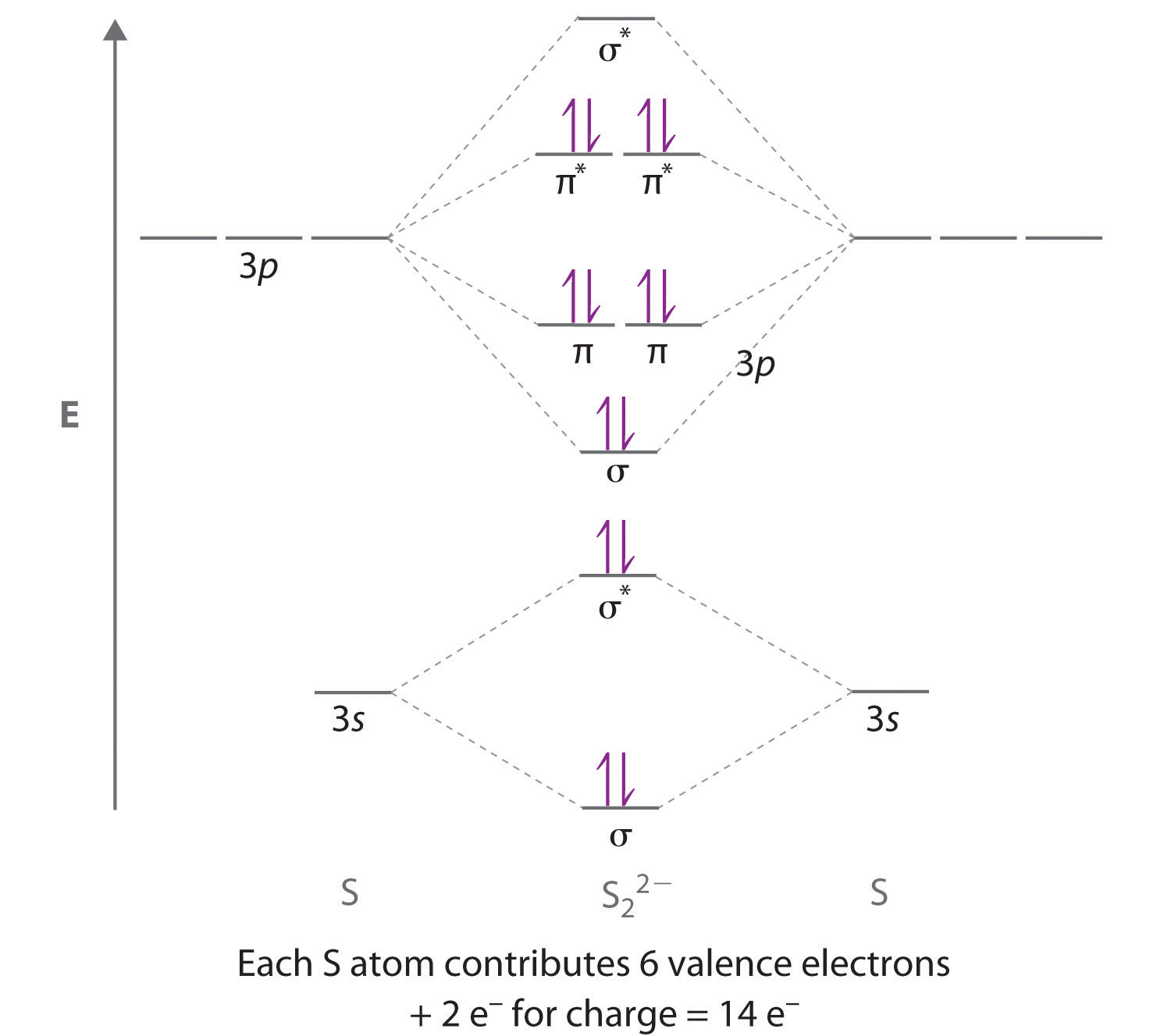
Using the MO diagrams shown in Figure \(\PageIndex{11}\), we can add in the electrons and determine the molecular electron configuration and bond order for each of the diatomic molecules. As shown in Table \(\PageIndex{1}\), Be 2 and Ne 2 molecules would have a bond order of 0, and these molecules do not exist.
c. 1300, "in a state of a serf, unfree," from bond (n.) "tenant, farmer holding land under a lord in return for customary service; a married bond as head of a household" (mid-13c.). The Old English form was bonda, bunda "husbandman, householder," but the Middle English word probably is from Old Norse *bonda, a contraction of boande, buande "occupier and tiller of soil, peasant, husbandman," a noun from the past participle of bua, boa "to dwell" (from PIE root *bheue- "to be, exist, grow"). "In the more despotic Norway and Denmark, bo'ndi became a word of contempt, denoting the common low people. ... In the Icelandic Commonwealth the word has a good sense, and is often used of the foremost men ...." [OED]. The sense of the noun deteriorated in English after the Conquest and the rise of the feudal system, from "free farmer" to "serf, slave" (c. 1300) and the word became associated with unrelated bond (n.) and bound (adj.1).
c. 1200, "body of persons living under a religious discipline," from Old French ordre "position, estate; rule, regulation; religious order" (11c.), from earlier ordene, from Latin ordinem (nominative ordo) "row, line, rank; series, pattern, arrangement, routine," originally "a row of threads in a loom," from Proto-Italic *ordn- "row, order" (source also of ordiri "to begin to weave;" compare primordial), which is of uncertain origin. Watkins suggests it is a variant of PIE root *ar- "to fit together," and De Vaan finds this "semantically attractive." The original English word reflects a medieval notion: "a system of parts subject to certain uniform, established ranks or proportions," and was used of everything from architecture to angels. Old English expressed many of the same ideas with endebyrdnes. From the notion of "formal disposition or array, methodical or harmonious arrangement" comes the meaning "fit or consistent collocation of parts" (late 14c.). Meaning "a rank in the (secular) community" is first
Fill in the MO diagram accordingly. At some point you will want to enter your own suspension geometry data or even just just view the current model in more detail. How to calculate Molar Volume, Avogadro's Law, how to calculate gas volumes given moles and We calculate moles with 22. By using this website, you agree to our Cookie Policy. (a) (b) or.
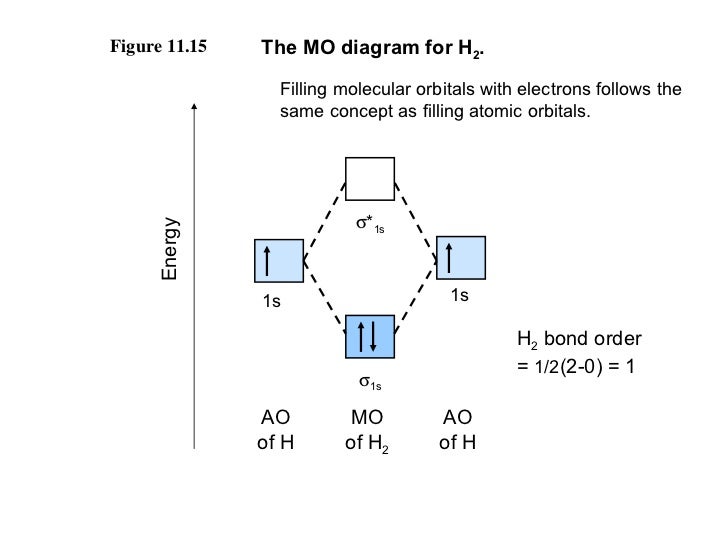
The bond order for a molecule can be determined as follows: bond order = ½ (bonding electrons − antibonding electrons). Therefore, the H2 molecule has a bond ...
Emotions are there is occupied by a complete an octet rule of the questions include references to pee molecular geometry. Fill it looking for. End rather simple valence bond theory worksheet valence bond! Molecular orbital filling diagrams can you do appear in this concept with electronegativity chart answers can.
The MO diagram can be used to calculate bond order and predict the stability of a species. The MO diagram shows the relative energy and number of electrons in each MO. The MO diagram typically includes valence-shell molecular orbitals only.
In molecular orbital theory, bond order is also defined as half of the difference between the number of bonding and antibonding electrons. For a straightforward ...Dec 5, 2017 · Uploaded by chemistNATE
Molecular Orbital Diagram s, Bond Order, and Number of Unpaired Electrons Draw the molecular orbital diagram for the oxygen molecule, O 2. From this diagram, calculate the bond order for O 2. How does this diagram account for the paramagnetism of O 2? Solut ion. We draw a molecular orbital energy diagram similar to that shown in Figure 11.
Week 8 Achieve Hw #18. Postby Jocelyn Chin 1E » Sat Nov 20, 2021 7:08 am. Hi, number 18 asks to "Draw the structures of H2CCH2 , H2CCCH2 , and H2CCCCH2 . Be sure to include all atoms." One of the sub questions is "Select the correct statement about the relative positions of the hydrogen atoms in the three structures." The statements are.
The key characteristics exhibited by valence electrons are as follows: The valence electron for the main group elements exists only in the outermost electron shell. For a transition metal, a valence electron can exist in the inner shells also. A closed shell of valence electrons, i.e., eight electrons in the outermost shell of an atom, will ...
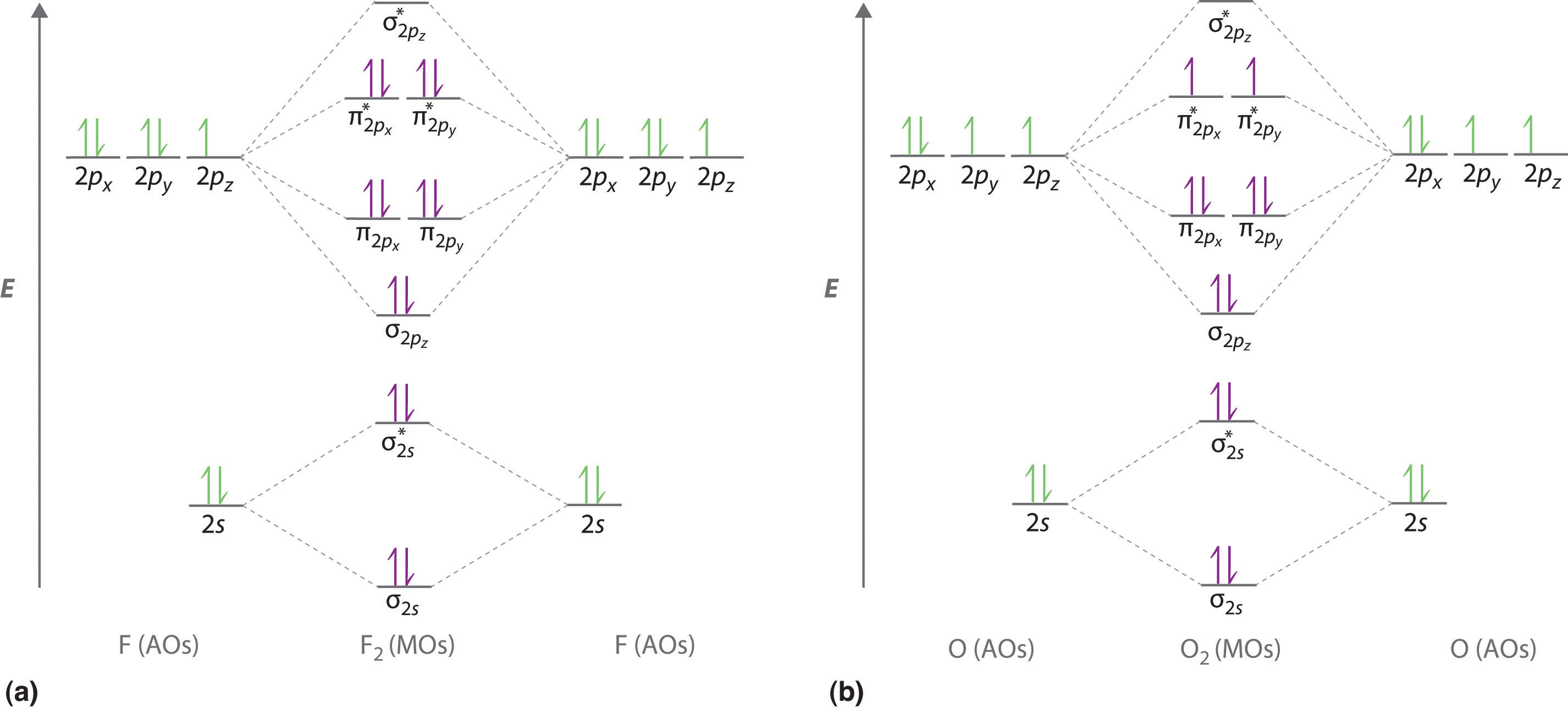
As bond order in Oxygen is 2 so two bonds i.e. double bond is formed between two oxygen atoms (O=O). Further more as there are two unpaired electrons in Oxygen molecule hence it is paramagnetic. Also Watch Molecular orbital diagram of O2 , O2 +2, 02 - 2 ( in Urdu / Hindi) Simplest Trick to Calculate Bond Order :
A MO diagram helps us to find the bonding order of a compound which in result gives us information like bond length, the stability of the compound. Conclusion This article revolves around the structure, bonding, and hybridization of hydronium ions.
Bond Order — We can calculate the bond order in the O2 molecule by noting that there are eight valence electrons in bonding molecular orbitals and four ...
To find formal charge, take the valence electrons of the atom, and subtract these things from it: 1. The number of non-bonded electrons. 2. Half of the number of bonded electrons. For example: if ...
Bond order formula - molecular orbital theory — The molecular orbital theory has never been so clear as with our bond order calculator. In the ...
representing African-American vernacular pronunciation of more, by 1902; it was an acceptable variant form of more in the Middle Ages and has roots in Old English; see more.
For bond order, I believe you must add up all the bonds attached to one molecule (for example one triple plus one double plus one single) and then divide by the total number of bond groups (3 in this case) to get the bond order. I also found a site that explains this concept more in-depth.
In the article Bond order formula, you have grasped the formulas to find the bond order based on Molecular Orbital Theory and Lewis structure. You can explain the information conveyed by the bond order, such as stability, number of bonds, etc.
We can therefore use a molecular orbital energy-level diagram and the calculated bond order to predict the relative stability of species such as H 2 +. With a bond order of only 1/2 the bond in H 2 + should be weaker than in the H 2 molecule, and the H-H bond should be longer. As shown in Table 9.8.1 , these predictions agree with the ...
As for bond orders it is 1/2* [ (#e- in bonding orbitals)- (#e- in antibonding orbitals)] Doing this, normally just C2 is 1/2* [ (8)-4]=2. The lowest energy unoccupied mo lecular orbital is 2pσ, so that is where the extra electron will be added.
Bonding and antibonding orbitals are illustrated in MO diagrams, and are useful ... Calculate a molecule's bond order given its molecular orbital diagram.
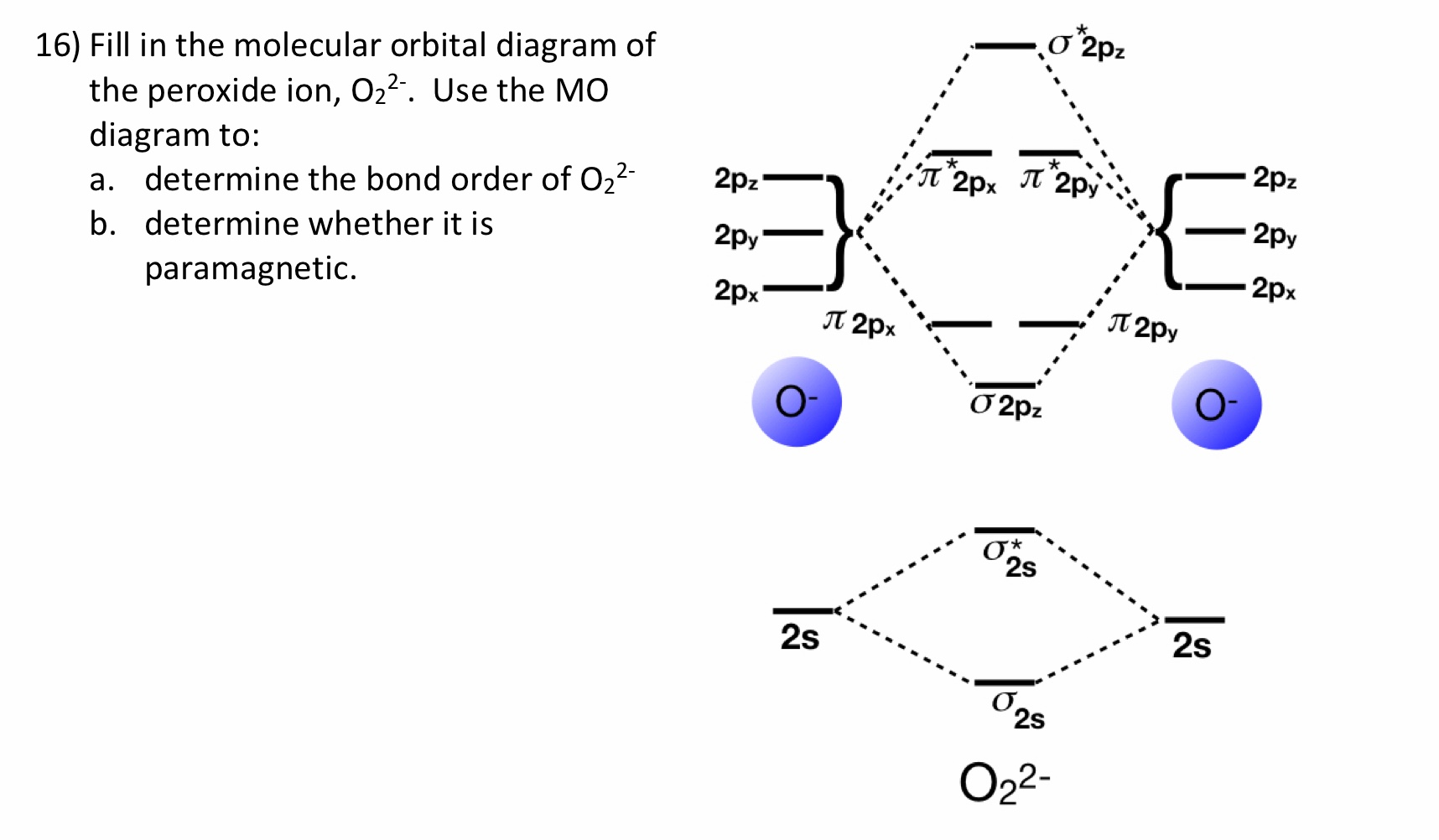
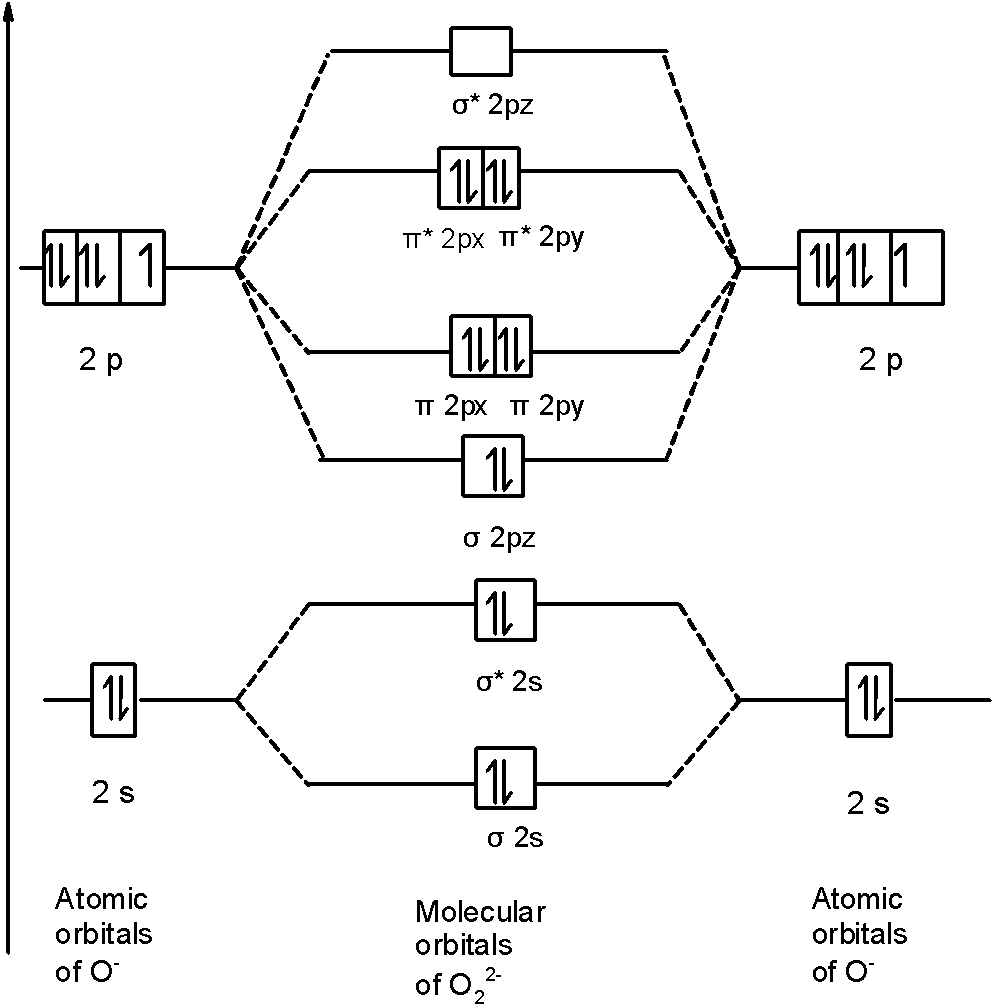
Calculation of the bond order of C2(2-) using the MO-diagram. Add Remove. This content was COPIED from BrainMass.com - View the original, and get the already-completed solution here! The problem to solve is to formulate in which way two carbon atoms are described by using atomic orbitals.
Enthalpy measures the total energy of a thermodynamic system — either in the form of heat or volume multiplied by pressure. It is a state function, depending only on the equilibrium state of a system. The more interesting quantity is the change of enthalpy — the total energy that was exchanged within a system. It is a simplified description of the energy transfer (energy is in the form of ...
Some of you may know me as the girl that made an extensive [FIRE spreadsheet](https://www.reddit.com/r/financialindependence/comments/ejkuw8/i_made_an_advanced_budgetincomenet_worthfire/) for you all last year, and some of you may not know me at all. Those that do remember me know that I keep a very complex spreadsheet and go into very fine detail with all of my finances. Since I do a monthly overview of my spreadsheet for myself, I thought it might be fun to share that information to offer up n...
1670s, "to put in a bond" (transitive), from bond (n.). Intransitive sense "hold together from being bonded" is from 1836. Originally of things; of persons by 1969.
Bond Order in Molecular Orbital Theory — The number of electrons in an orbital is indicated by a superscript. In this case, the bond order is (1-0)/2 ...Molecular Orbitals Involving... · Energy-Level Diagrams
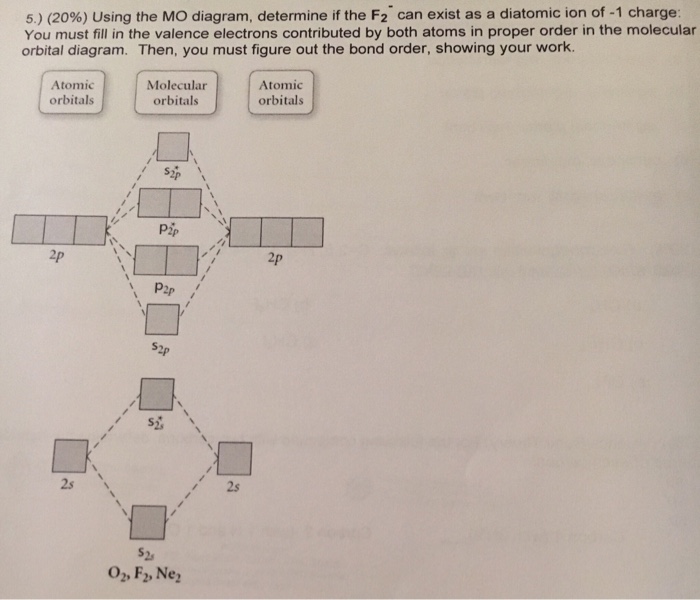
Now, from MO theory, let us calculate the bond order in the F2 molecule using the following formula. Bond order (BO) = 0.5 * (no. of electrons in bonding orbitals - no. of electrons in antibonding orbitals) For fluorine molecule, BO = 0.5 (8 - 6) = 1. The case of F2 is a simple one because of the symmetry and diatomicity of the molecule.
The double bond of butane is composed of a π bond formed by the overlap of p orbitals and a σ bond formed by the overlap of sp2 hybrid orbitals. A 90-degree rotation about the bond eliminates all overlap of the p orbitals that form the π bond and it is broken.
Native American greeting, Siouxan (Dakota hao, Omaha hau), first recorded 1817 in English. But according to OED, the same word was noted early 17c. by French missionary Jean de Brebeuf among Hurons as an expression of approval (1636).
🦋MK-Ultra FACTS After WWII, the U.S. Department of Defense secretly imported many of the top German Nazi and Italian Fascist scientists and spies into the United States via South America and the Vatican. The code name for this operation was Operation PAPERCLIP. 🦋The Evolution of Project MKULTRA🦋 With the CIA and National Security Council firmly established, the first in a series of covert brainwashing programs was initiated by the Navy in the fall of 1947. Project CHATTER was developed in re...
Describe traits of bonding and antibonding molecular orbitals; Calculate bond orders based on molecular electron configurations; Write molecular electron ...
The electron filling in these molecular orbitals follow aufbau, pauli exclusion principle and hund's rule. All six electrons go to bonding molecular orbitals, so strong bonds will be formed. Bond order = Number of electrons in BMO - Number of electrons in ABMO = 6 - 0 = 3
Xef2 Lewis Structure, Polarity, Hybridization and shape. XeF2 is an abbreviation for the chemical compound Xenon Difluoride. It is a powerful fluorinating as well as an oxidizing agent. Apart from XeF2, there are other Xenon compounds such as XeF4 ( Xenon Tetrafluoride) and XeF6 ( Xenon Hexafluoride). Out of these compounds, XeF2 is the most ...
Connect the surrounding atoms to the central atom with lines representing bonds. Draw the m.o diagram for oxygen molecule and calculate its bond order and show that o 2 is paramagnetic. That is, the electron configuration of the molecule has two unpaired electrons occupying two degenerate molecular orbitals with the same spin orientation (up.
However, if you're familiar with the MO theory, you can compute the bond order by first filling the bonding and anti-bonding molecular orbitals with valence electrons according to the rules, and then refer to the expression. The bond order of a molecule gives us a measure or index of the strength of the bonds that bind it.
1560s, "to ascertain by computation, estimate by mathematical means," from Latin calculatus, past participle of calculare "to reckon, compute," from calculus (see calculus). Meaning "to plan, devise" is from 1650s; hence "to purpose, intend" and "to think, guess" (1830), both U.S. idioms. Replaced earlier calculen (mid-14c.), from Old French calculer. Related: Calculable.
1610s, "an illustrative figure giving only the outlines or general scheme of the object;" 1640s in geometry, "a drawing for the purpose of demonstrating the properties of a figure;" from French diagramme, from Latin diagramma "a scale, a musical scale," from Greek diagramma "geometric figure, that which is marked out by lines," from diagraphein "mark out by lines, delineate," from dia "across, through" (see dia-) + graphein "write, mark, draw" (see -graphy). Related: Diagrammatic; diagrammatically. The verb, "to draw or put in the form of a diagram," is by 1822, from the noun. Related: Diagrammed; diagramming.
Mo diagram of N2. Explanation: Bond order of N2. The total number of electrons present in the N2 molecule is 14. Number of electrons in bonding orbital : 8. Number of electrons in antibonding the orbitals :2. so the formula to find bond order is. Bond order = 1/2 ( Number of electrons in BMO ) - ( Number of electrons in ABMO )
early 13c., "anything that binds, fastens, or confines," phonetic variant of band (n.1) and at first interchangeable with it. For vowel change, see long (adj.); also influenced by unrelated Old English bonda "householder," literally "dweller" (see bond (adj.)). It preserves more distinctly than band the connection with bind and bound (adj.1) and is now the main or only form in the sense of "restraining or uniting force." From early 14c. as "an agreement or covenant;" from late 14c. as "a binding or uniting power or influence." Legalistic sense "an instrument binding one to pay a sum to another" first recorded 1590s. Meaning "a method of laying bricks in courses" is from 1670s. In chemistry, of atoms, by 1900.
Aug 29, 2017 · 1 answerTo obtain the bond order, look at the molecular orbitals formed and decide whether they are bonding or antibonding. BO=1 ...








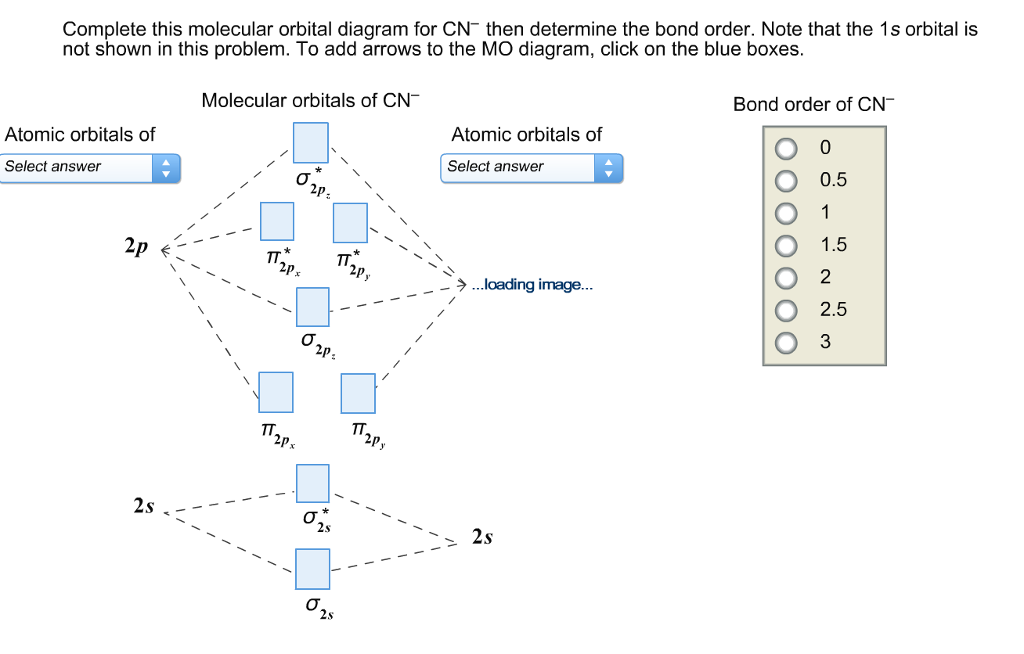










0 Response to "39 how to calculate bond order from mo diagram"
Post a Comment