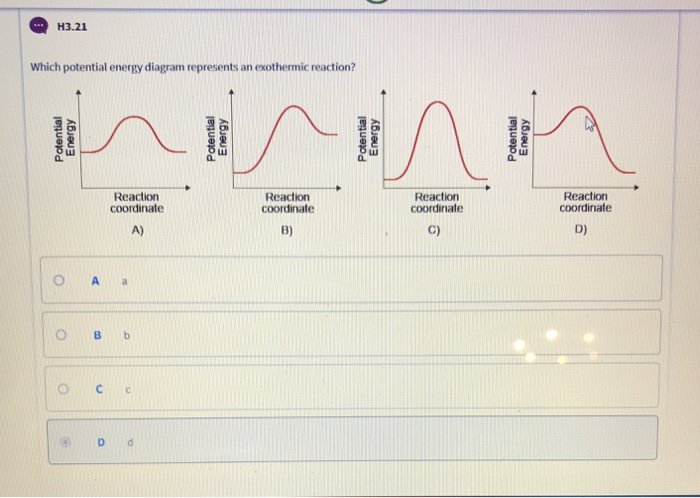
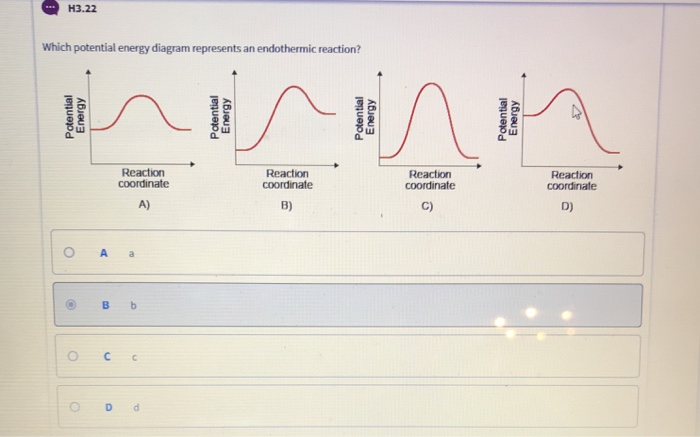
39 an energy diagram that shows the reactants having greater energy than the products illustrates an:
An energy diagram that shows the reactants having greater energy than the products illustrates an: asked Sep 18, 2016 in Chemistry by FSU_Gal. A) endothermic reaction. B) exothermic reaction. C) isothermic reaction. D) impossible reaction. E) none of the above. general-chemistry
6. An energy diagram that shows the reactants having greater energy than the products illustrates an: An energy diagram that shows the reactants having greater energy than the products illustrates an: impossible reaction. endothermic reaction. isothermic reaction. exothermic reaction. none of the above. 7. Which among the following statements ...
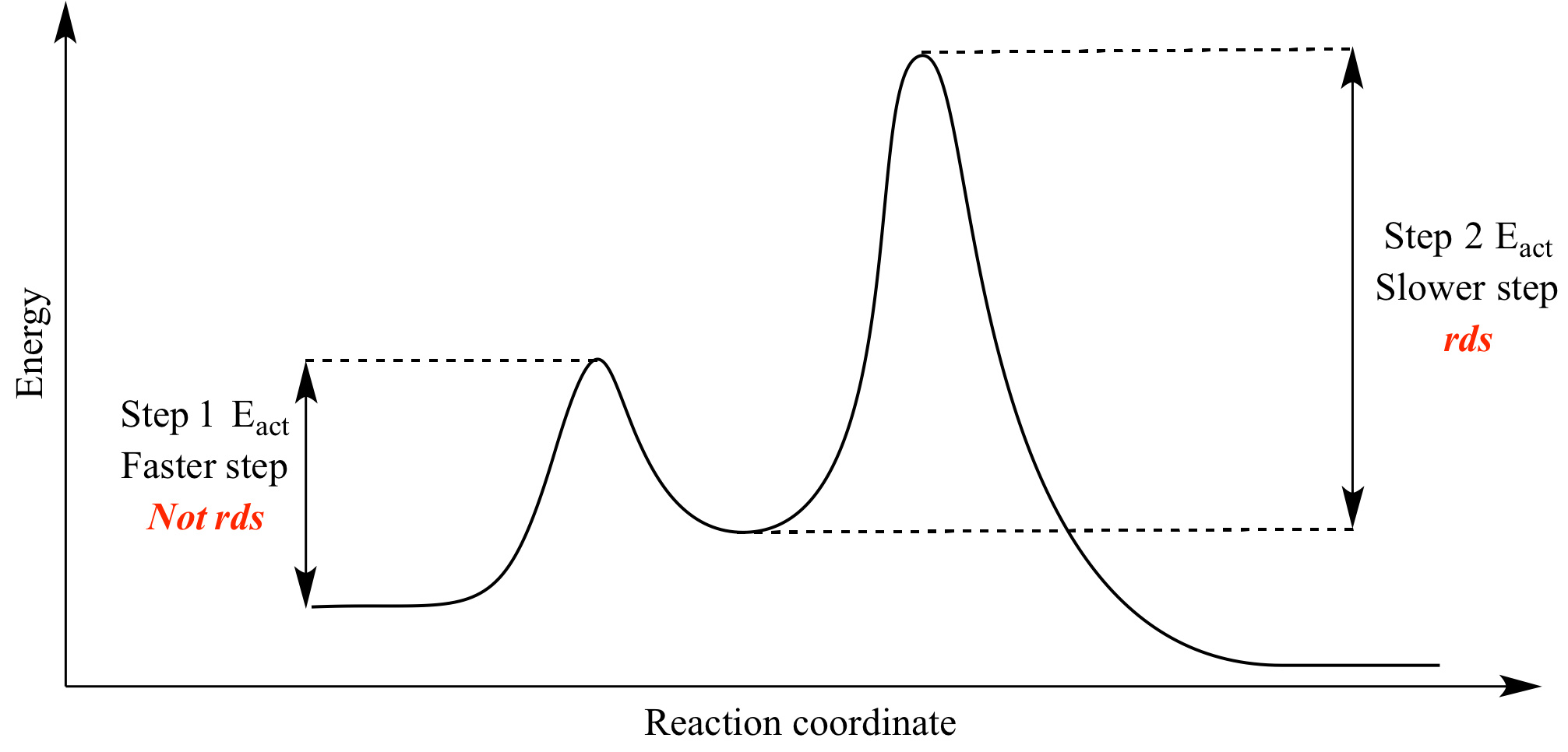
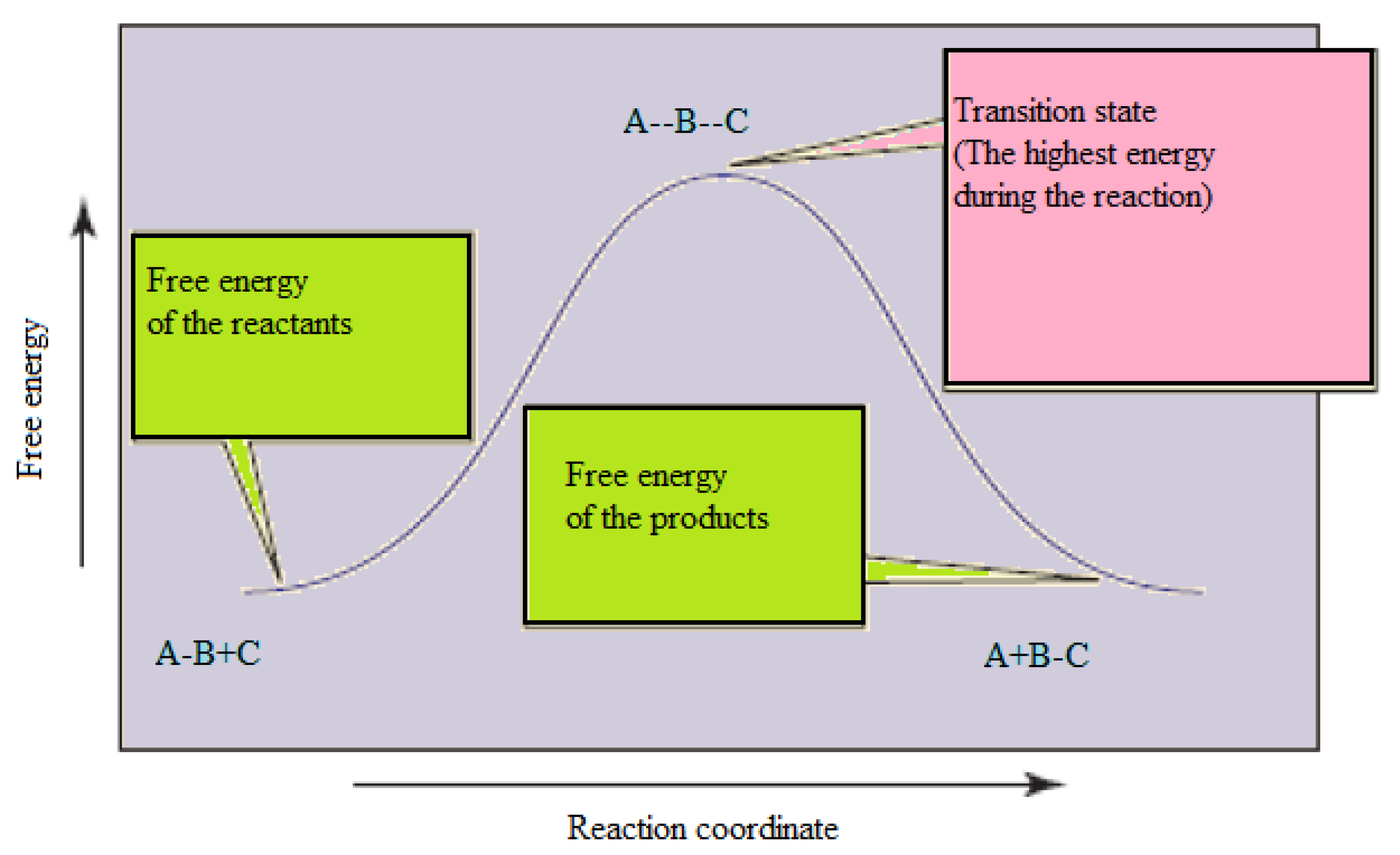
Typically, we envision reactions proceeding left to right along the reaction coordinate, so often, the activation energy is only noted for the forward reaction. The activation energy on the diagram below shows the barrier to be 102.6 kJ mol-1. Barriers are measured in energy per mole (typically kJ mol-1).
An energy diagram that shows the reactants having greater energy than the products illustrates an:
Problem: An energy diagram that shows the reactants having greater energy than the products illustrates what type of reaction? FREE Expert Solution. Recall: Exothermic: E reactants > E products. Endothermic: E reactants < E products. 86% (56 ratings) Problem Details.
In Diels-Alder terminology the two reactants are referred to as the diene and the dienophile. The following diagram shows two examples of [4 π +2 π] cycloaddition, and in the second equation a subsequent light induced [2 π +2 π] cycloaddition. In each case the diene reactant is colored blue, and the new σ-bonds in the adduct are colored red.
an energy diagram that shows the reactants having greater energy than the products illustrates an exothermic reaction if a solid piece of shiny sodium metal is exposed to chlorine gas, a large amount of heat is released and the white solid sodium chloride (table salt) forms. based on this information which of the following statements is true?
An energy diagram that shows the reactants having greater energy than the products illustrates an:.
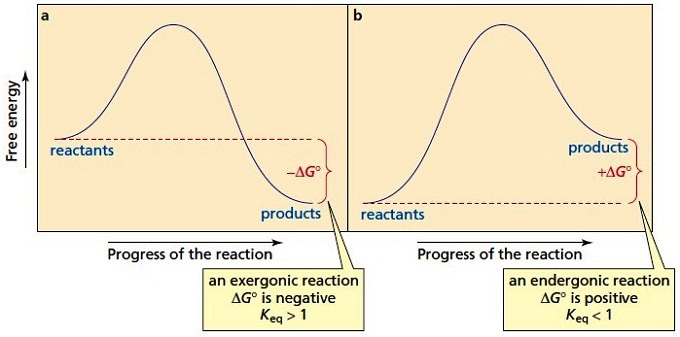
Figure 1. A potential energy diagram shows the total potential energy of a reacting system as the reaction proceeds. (A) In an endothermic reaction, the energy of the products is greater than the energy of the reactants and ΔH is positive. (B) In an exothermic reaction, the energy of the products is lower than the energy of the reactants and ...
Let's look at the elements of this enthalpy diagram. First, as noted, the y -axis is labeled 'enthalpy' and the x -axis is labeled 'reaction progress.'. Then we have the actual energy diagram plot ...
Energy level diagrams for endothermic reactions In endothermic reactions the reactants have a less energy than the products . The difference between these two energy levels is the energy absorbed from the surroundings. It is represented in an energy level diagram as a vertical jump from a lower to a higher level - the bigger the difference,
Note that resonance stabilization of the allyl cation is greater than comparable stabilization of 1,3-butadiene, because charge is delocalized in the former, but created and separated in the latter. An explanation for the temperature influence is shown in the following energy diagram for the addition of HBr to 1,3-butadiene.
An energy diagram that shows the reactants having greater energy than the products illustrates an: exothermic reaction. Consider the following specific heats of metals. Metal Specific Heat (J/g∙°C) Aluminum 0.903 Copper 0.385 Gold 0.128 Iron 0.449 Silver 0.235
Energy Diagram. Q. Which reaction requires the least energetic collisions to reach the transition state/. Solved • Feb 19, 2020. Energy Diagram. Q. For the first-order reaction 1/2 N2O4 (g) → NO2 (g); ∆H = 28 kJ/molthe activation energy is 53 kJ/mol. What is the activation energy for the reverse ...
An energy diagram that shows the reactants having greater energy than the products illustrates an: a. endothermic reaction. b. impossible reaction. c. exothermic reaction. d. isothermic reaction. 248. What is the value of -25∘C on the Kelvin scale? a. -298
AS-Level REACTION KINETICS Q1(a) Draw a diagram to show the Boltzmann distribution of molecular energies.Label the axes. (b) Comment on the shape of the distribution curve. (c)(i) Explain the meaning of the term activation energy. (ii) Insert and label the activation energy in your diagram in (a). (d)(i) Use a dotted line added to your diagram in (a) to show how the distribution of
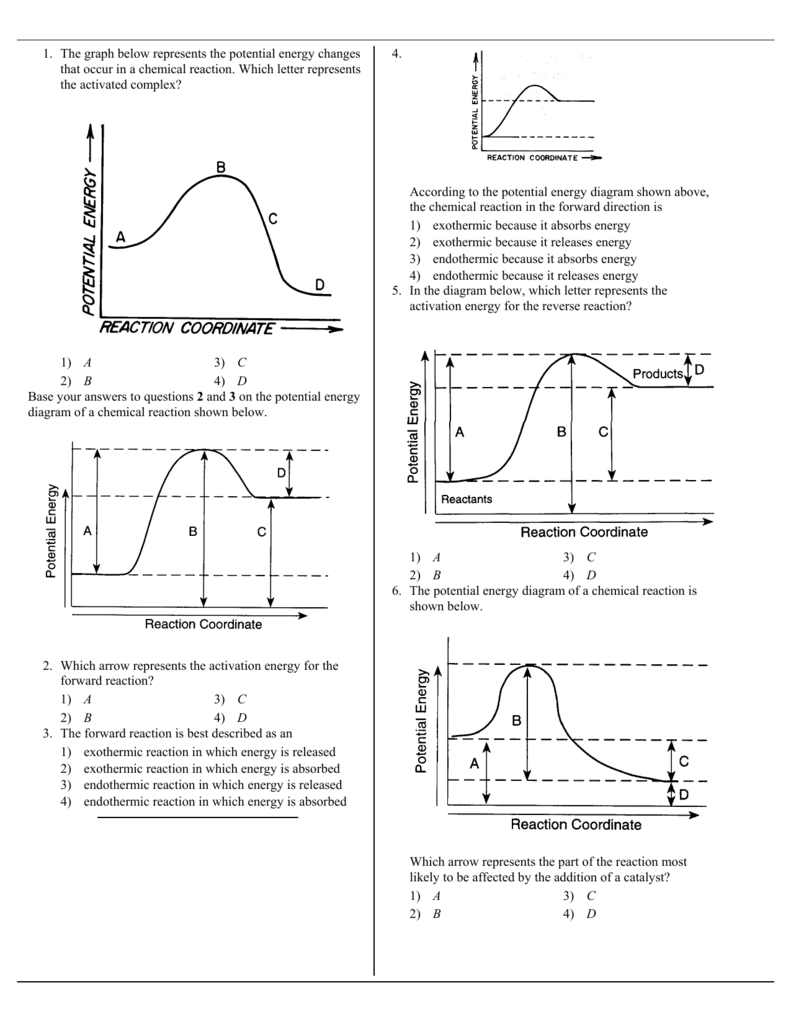
55. The potential energy diagram below shows the reaction X + Y ß à Z ... The amount of products is greater than the amount of reactants. (B) The amount of products is equal to the amount of reactants. ... (D) The sign of D H is negative, and the products have more potential energy than the reactants. 86. The ...
An energy diagram of a chemical reactions illustrates the changes of energy as the chemical reactions advances. At first, the energy in the diagram is the energy of the reactants. As the reaction goes forward, the reactants start to react forming a transition compound, with a maximum energy level on the graph. This is the hill.
An energy diagram shows the reactants having greater energy than the products illustrates what? This shows that the reaction is endothermic because energy is absorbed in the reaction and not produced.
An energy diagram that shows the reactants having greater energy than the products illustrates an: endothermic reaction. none of the above isothermic reaction. exothermic reaction. impossible reaction.
An energy diagram that shows the reactions having greater energy than they products illustrates an: A. Impossible reaction B. Exothermic reaction C. Endothermic reaction D. Isothermic reaction E. none of the above
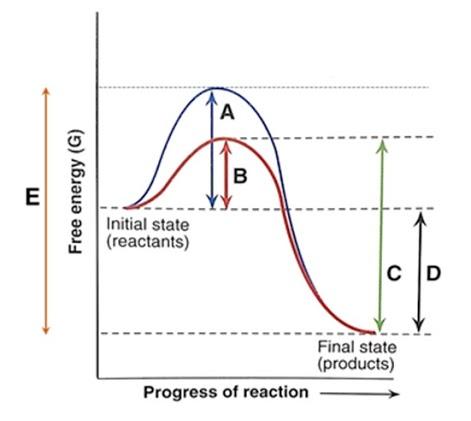
The potential energy of an activated complex is typically greater than the potential energy of the reactants and products. That is because some of the energy is already used.
Energy Diagram. Q. Draw a rough sketch of the energy profile for each of the following cases:b. ΔE = -10 kJ/mol, Ea = 50 kJ/mol. Solved • Nov 7, 2018. Energy Diagram. Q. The activation energy for some reaction X2 (g) + Y2 (g) → 2XY (g)is 167 kJ/mol, and ΔE for the reaction is 128 kJ/mol. What is the activation energ...
In an endothermic reaction, the reactants absorb heat energy from the surroundings to form products. Thus, the products formed have more energy than the reactans, H products > H reactants. Therefore, ΔH is positive. Energy level diagrams are used to shows the energy content of chemicals before and after a reaction. They show:
1. Identify the general shape of the energy diagram Energy should conserve for any chemical reaction. The reaction in question is exothermic (releases heat) hence its products shall have chemical potential energies lower than that of its reactants- some of the potential energies have been converted to thermal energy during the reaction process.
Energy is the single main component of the universe. Energy is the capacity to do work. All of the above statements are true. A solid form of matter in which there is long range repeating order is called _____. An energy diagram that shows the reactants having greater energy than the products illustrates an:
On the axis below, draw a potential energy diagram for the reaction. Label the reactants and the products on your graph. [Do not number the axis.] Draw an arrow on your Reaction Coordi nate gram to represent the heat of the reaction. Label the arrow AH. The potential energy diagram of a chemical reaction is shown below. 200 - 150 100 - 50 (a ...
An energy diagram that shows the reactants having greater energy than the products illustrates an: isothermic reaction. exothermic reaction. endothermic reaction. impossible reaction. none of the above. exothermic. In order, what is the freezing point, room temperature and boiling point of water according to the Celsius scale?
The overall change in energy in a reaction is the difference between the energy of the reactants and products. Exothermic reactions The diagram shows a reaction profile for an exothermic reaction.
Mar 10, 2009 · The energy storage capabilities of supercapacitors are substantially greater than that of conventional capacitors, by approximately two orders of magnitude (10–100 s kW) , . The major problems with capacitors, similar to flywheels, are the short durations and high energy dissipations due to self-discharge loss.
Sep 24, 2014 · Hydrogen production technologies. Hydrogen is considered to be an important energy carrier and storage media for a future hydrogen economy. Hydrogen offers a sustainable energy future for both transport and stationary applications with near zero greenhouse gas emissions especially when generated by splitting water and combining with renewable energy sources (solar, wind, ocean).
Nov 18, 2020 · In this hydrolysis reaction, the products contain less energy than the reactants; there is a release of energy (> 7 kcal/mol). One reason for the amount of energy released is that hydrolysis relieves the electron-electron repulsions experienced by the negatively charged phosphate groups when they are bonded to each other (Figure 11.3 ...
Net calorific value at constant volume is the absolute value of the specific energy of combustion, in joules, for unit mass of the biofuel burned in oxygen under conditions of constant volume and such that all the water of the reaction products remains as water vapour (in a hypothetical state at 0.1 MPa), the other products being as for the ...
73) An energy diagram that shows the reactants having greater energy than the products illustrates an 73) A) exothermic reaction. B) isothermic reaction. C) impossible reaction. D) endothermic reaction. 74) If a solid piece of shiny sodium metal is exposed to chlorine gas, a large amount of heat is
the reactants have less potential energy than do the products. Energy must be input in order to raise the particles up to the higher energy level. Energy + Reactants--> Products. Exothermic Reactions. the reactants have more potential energy than the products have. The extra energy is released to the surroundings. Reactants --> Products + Energy
An energy diagram shows the reactants having greater energy than the products illustrates what? This shows that the reaction is endothermic because energy is absorbed in the reaction and not produced.
An energy diagram that shows the reactants having greater energy than the products illustrates an. When the value 4449 is rounded to two significant figures the number should be reported as. 4 the curve diagram shows that and the choice c indicates correctly the change in the energy for an endothermic reaction.





















0 Response to "39 an energy diagram that shows the reactants having greater energy than the products illustrates an:"
Post a Comment