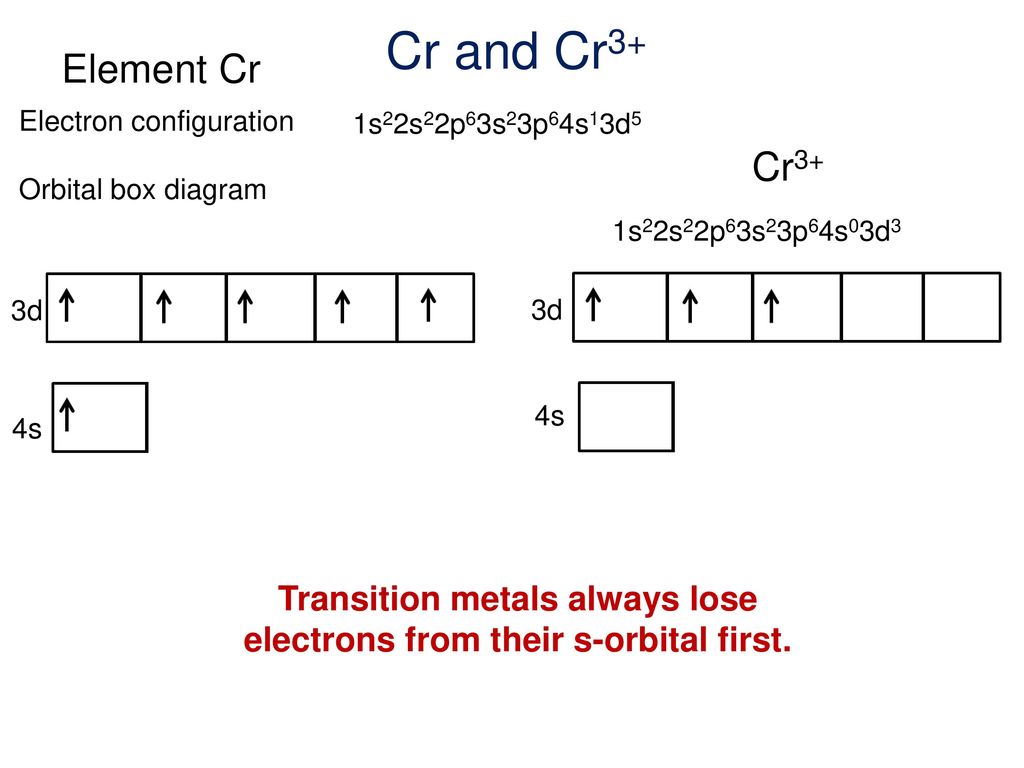
37 cr3+ orbital diagram
Prof. Marcetta Y. Darensbourg myd@mail.chem.tamu.edu 408 Chemistry Bldg. (979) 845-5417 Office Hours: Th 11-12; M 11-12. Or any afternoon · Admin. Coord.: Abbey Kunkle darensbourg_asst@chem.tamu.edu 407 Chemistry Bldg. (979) 845-5417
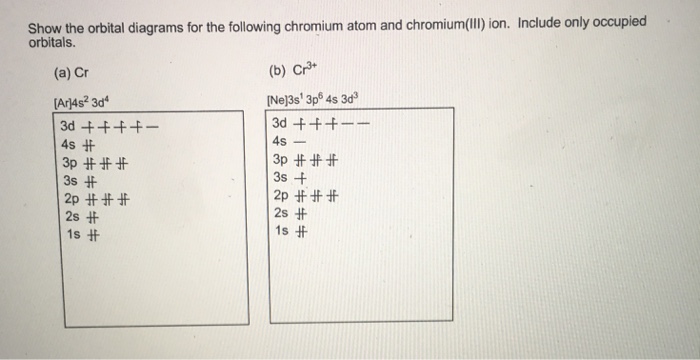
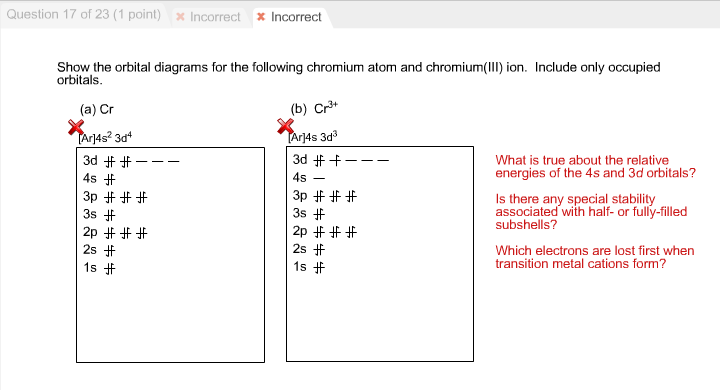
Write orbital diagrams for each of these ions. V5+ Cr3+ Ni2+ Fe3+ Determine if the ion is diamagnetic or paramagnetic. [Ne] 3s^2 3p^6 [Ar] 4s^0 3d^3 [Ar] 4s^0 3d^8 [Ar] 4s^0 3d^5 diamagnetic: V5+ paramagnetic: Cr3+, Ni2+, Fe+. Choose the larger atom from each of the following pairs. Al or In Br or Ar S or Sn Si or Cl. In Br Sn Si. Choose the ...
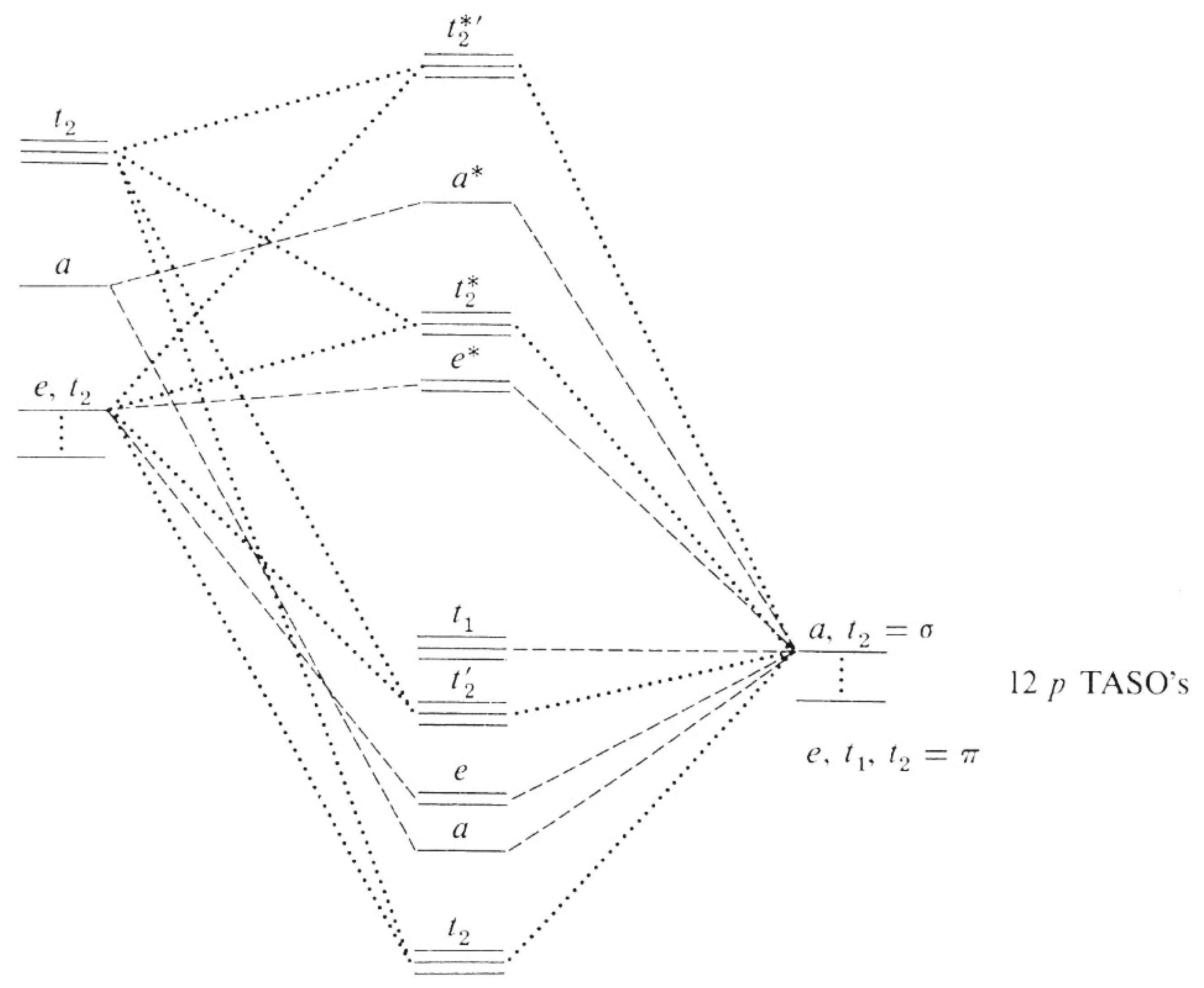
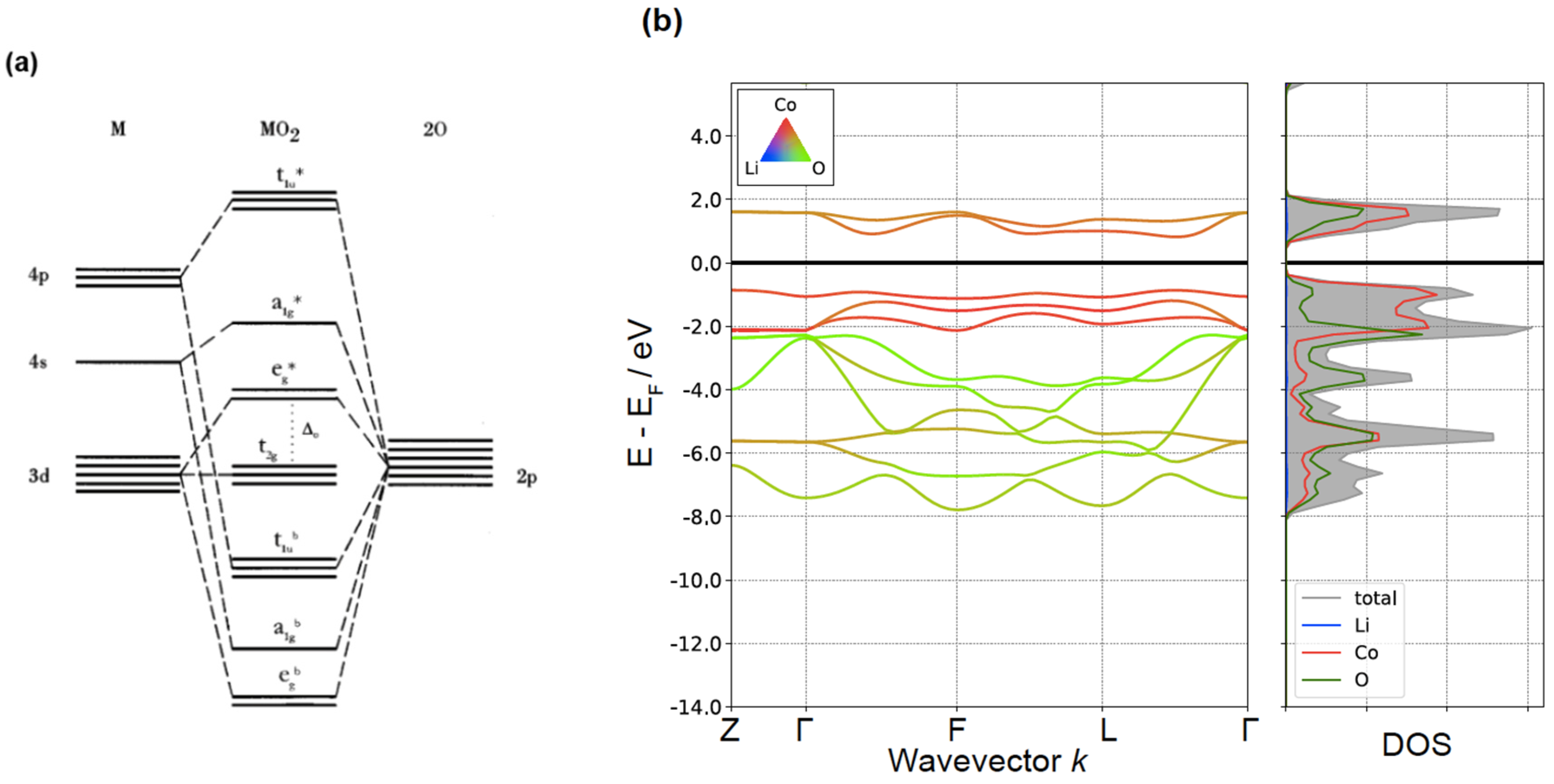
ions in tetrahedral fields, only that the diagram for a 3d10-N ion has to be utilized. In addition, the crystal field strength is reduced from its octahedral value. For instance, a 3d3 ion in tetrahedral symmetry has an identical diagram as a 3d7 ion in octahedral symmetry with the reduced value of D q. The parameters D q

Cr3+ orbital diagram
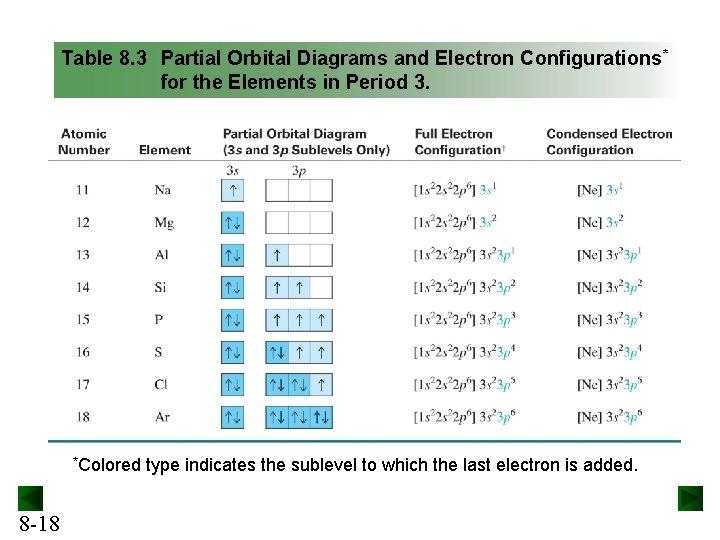
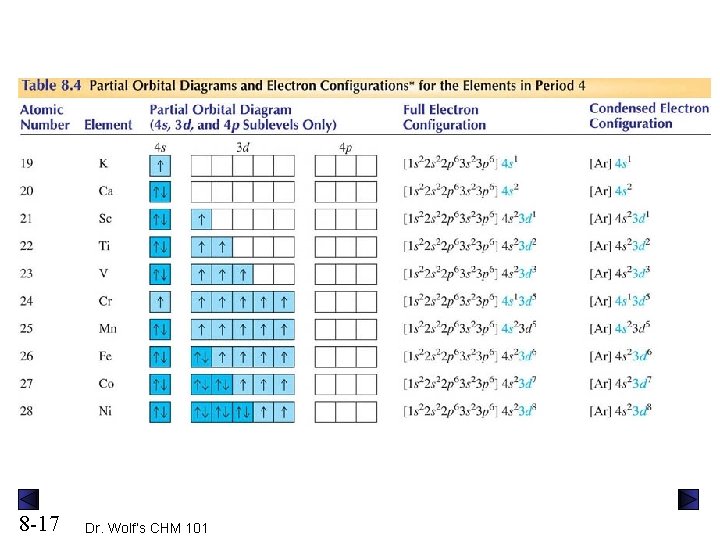
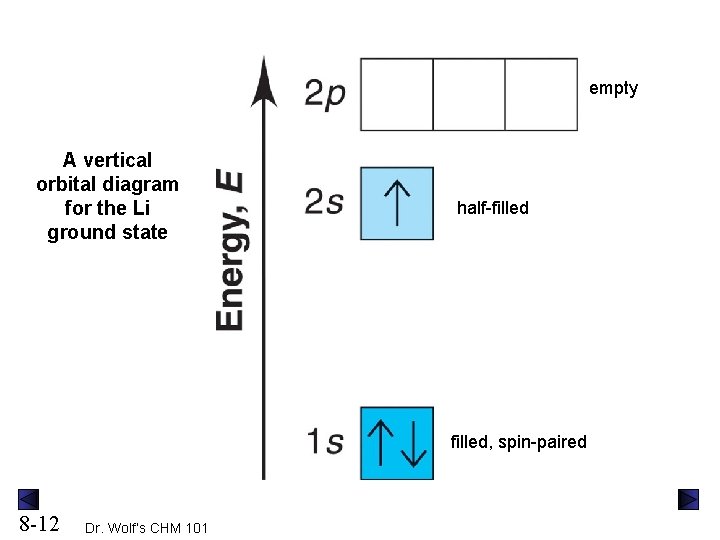
Orbital Diagrams. Another way to represent the order of fill for an atom is by using an orbital diagram often referred to as "the little boxes": The boxes are used to represent the orbitals and to show the electrons placed in them. The order of fill is the same but as you can see from above the electrons are placed singly into the boxes before ...
So the octahedron from crystal field splitting takes place like this two off. The orbital's going higher and adjusted, and in the ground state, and three of them come to a lower energy state and then the ground state. We call them Tito cheer pickles. And this as each your vittles. So for chromium three plus, we have for chromium. We know the chromium silica reconfiguration is like this are ...
Question: Write orbital diagrams for each of these ions. V5+,Cr3+,Ni2+,Fe3+ Determine if the ion is diamagnetic or paramagnetic. V5+,Cr3+,Ni2+,Fe3+ This problem has been solved! ... Write orbital diagrams for each of these ions.
Cr3+ orbital diagram.
April 18, 2021 - Chromium is a transition metal and it has 24 electrons and here is the orbital diagram. If we're going to make this short hand and make the electron configuration for this we would make this 1s2, 2s2, 2p6, 3s2, 3p6, 4s2, 3d4 okay from now on every time you see 3d4 you're going to change it, ...
Tomorrow's answer's today! Find correct step-by-step solutions for ALL your homework for FREE!
The valence electron configuration of Cr is 1s2, 2s2, 2p6, 3s2, 3p6, 3d5, 4s1 instead of 1s2, 2s2, 2p6, 3s2, 3p6, 3d4, 4s2 because one of the electron from the s orbital jumped to the d orbital. By distributing its electrons along the empty orbitals, it becomes more stable. Since the ground state for Cr is 1s2, 2s2, 2p6, 3s2, 3p6, 3d5, 4s1.
Both of the configurations have the correct numbers of electrons in each orbital, it is just a matter of how the electronic configuration notation is written ( ...5 Jul 2019 · Uploaded by Wayne Breslyn
Orbital Diagram. Orbital diagrams are ways to assign electrons in an atom or ion. Each atomic orbital is represented by a line or a box and electrons in the ...1 answer · Top answer: 1. The orbital diagrams are shown in the figures below. 2. V5+V5+ has all the electrons paired up so it is...
After the 4s is full we put the remaining six electrons in the 3d orbital and end with 3d9. Therefore the expected electron configuration for Copper will be 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 9 . Note that when writing the electron configuration for an atom like Cu, the 3d is usually written before the 4s.
April 29, 2018 - Answer: Go to the following website to find the orbital diagram of any element of any Oxidation state. Orbital Energy Diagram and Atomic Electron Configuration Tool As for an actual diagram (per Wiki) is above. You can also find the electron configuration on both websites. For an overview and ...
In order to continue enjoying our site, we ask that you confirm your identity as a human. Thank you very much for your cooperation
To write the configuration for the Chromium ions, first we need to write the electron configuration for just Chromium (Cr). We first need to find the number...
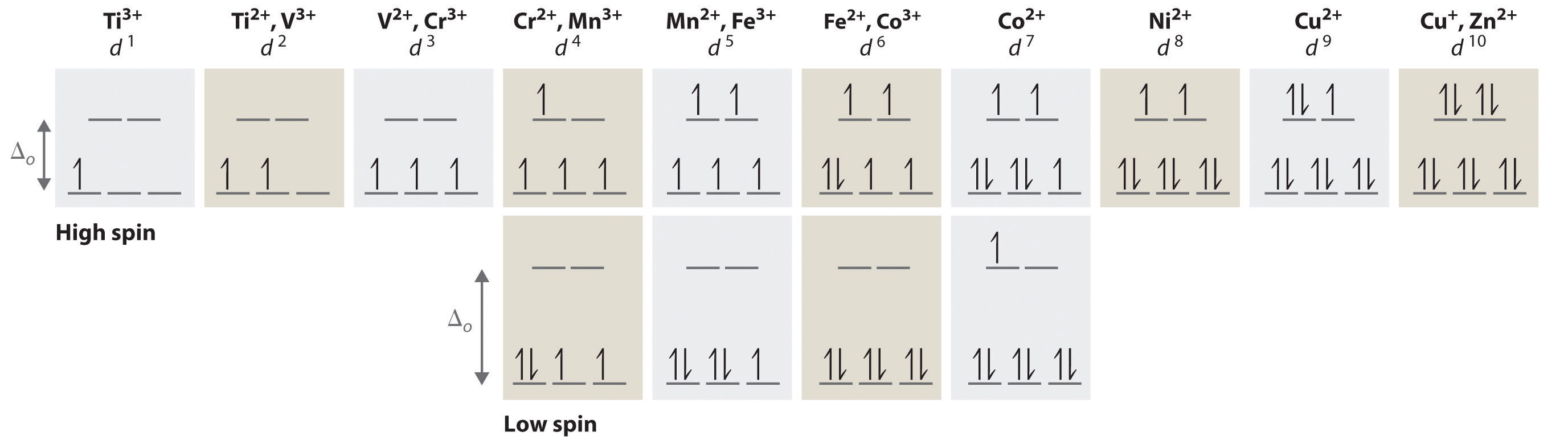
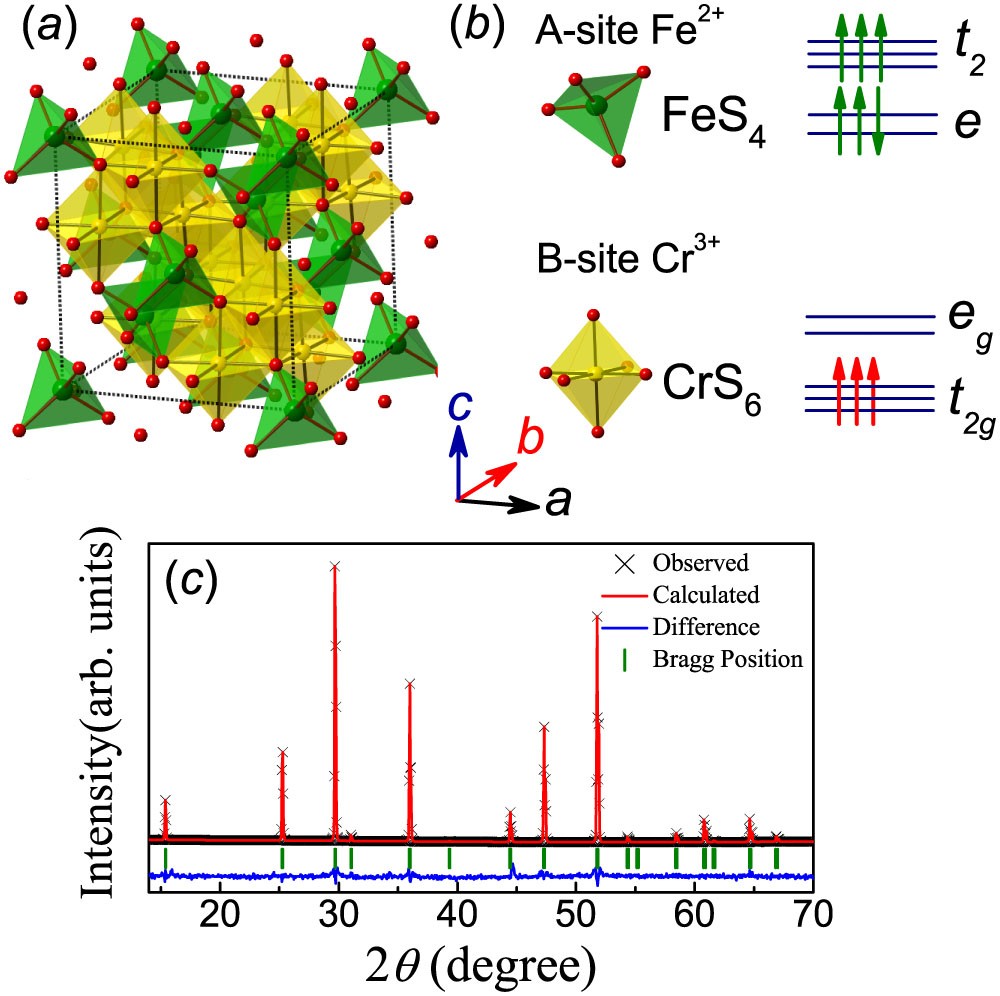
Constructing an orbital energy diagram, considering Aufbau's Principle and Hunds Rule it can be found that Cr3+ has 3 paired and 1 unpaired in its d-she'll and Fe2+ has 3 paired. See Crystal Field Theory (CFT), high/low spin, ligand field theory and the spectrochemical series - these will broaden your understanding 7.6K views View upvotes K G
electrons into the same orbital •Πeis a stabilizing energy for electron exchange associated with two degenerate electrons having parallel spin total 3 e 0 c eg* t2g d4HS eg* t2g d8 eg* t2g d6LS total 7 e 3 c total 6 e 3 c LFSE 3 0.4 O 10.6 O 0.6 O LFSE 6 0.4 O 20.6 O
November 1, 2015 - Z for Cr is 24. Cr(VI) therefore has 18 electrons to distribute Cr metal is 1s^(2)2s^(2)2p^(6)3s^(2)3p^(6)4s^(1)3d^(5) Cr(VI) has all the valence electrons removed; 18 electrons to distribute, i.e. 1s^(2)2s^(2)2p^(6)3s^(2)3p^(6)
Transition Fe3+ ions and draw the orbital box diagrams for both ions. Using this. There for 1s2 2s2 2p6 3s2 3p6 3d5 is the electronic configration for Fe3+. half of electrons (there must be one electron in each orbital, and d has 5 orbitals). That's for filling up orbitals for ground state atoms.

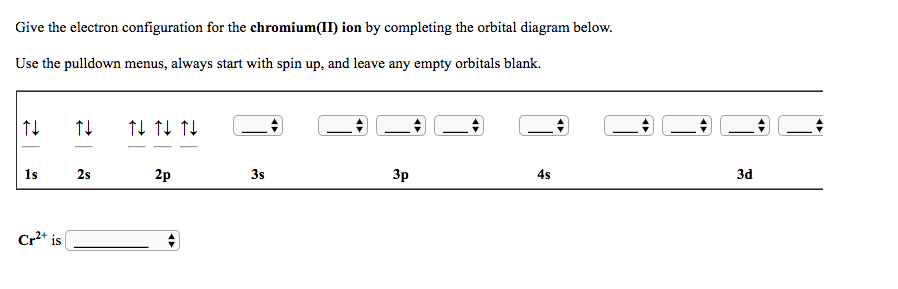
Fill in the energy orbital diagram for the following atom cr(iii). be sure to label orbitals by n and l quantum numbers, and place electrons in the appropriate energy level. you will not fill all
Chromium Electron Configuration. Mr. Causey shows you step by step how to write the electron configuration and orbital notation for chromium (Cr). Remember t...
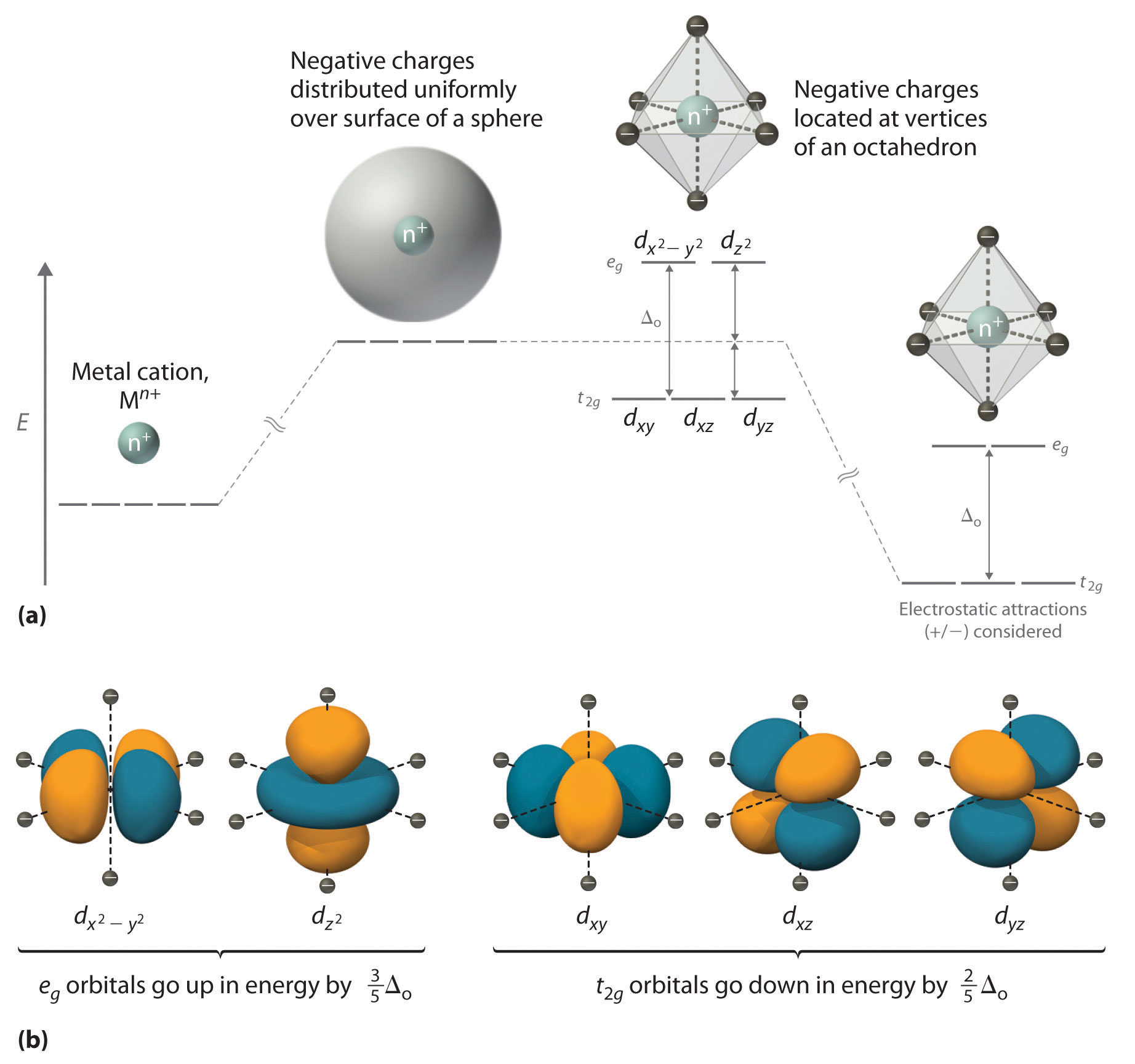
an crystal field splitting diagrams to show orbital occupancies in both weak and strong octahedral fields, and (ii) indicate the number of unpaired electrons in each case. Label . the diagrams (iii) weak or strong field, (iv) high spin or low spin (as appropriate), (v) with the names of the d-orbitals, and (vi) with the appropriate orbital sets ...
orbital type as a superscript, e.g. 1s22p1 would denote an atom with 2 electrons in its 1s orbital, and one in the 2p orbital. The ground state con guration is the lowest-energy con guration. Marc R. Roussel Multielectron atoms September 14, 2018 11/23
This WebElements periodic table page contains properties of free atoms for the element chromium
Note that it is 4s13d5 and not 4s23d4 because a half filled d orbital is more stable than a partially filled d orbital. However, the chromium ion Cr3+ possesses 24e− −3e− = 21e− due to the loss of 3 of its electrons. Thus, the electron configuration of Cr3+ is: Cr3+:1s22s22p63s23p64s03d3. Answer link.
Orbital diagram for cr3+. The electronic configuration of Cr having atomic number of 24 is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 1 3d 5 which is half-filled d-orbital.. Cr3+ has 3 electrons removed from the outermost shell. Therefore, the electronic configuration comes out to be [Ar]3d3.
Answer: Go to the following website to find the orbital diagram of any element of any Oxidation state. Orbital Energy Diagram and Atomic Electron Configuration Tool As for an actual diagram (per Wiki) is above. You can also find the electron configuration on both websites. For an overview and ...
3. 3+Using your answer from Question 2, draw a d-orbital splitting diagram for low-spin Co similar to the one given for Cr3+ 3+in the introduction to this experiment. Almost all Co complexes are low-spin, with a minimum number of unpaired electrons. (Refer to your text for further explanation.) 4.
Our videos prepare you to succeed in your college classes. Let us help you simplify your studying. If you are having trouble with Chemistry, Organic, Physics, Calculus, or Statistics, we got your back! Our videos will help you understand concepts, solve your homework, and do great on your exams.
Chem4Kids.com! Chromium atomic orbital and chemical bonding information. There are also tutorials on the first thirty-six elements of the periodic table.
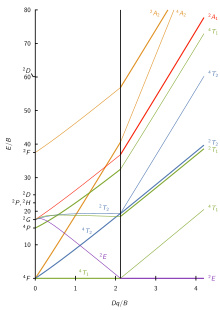
Chem242. Int. Inorg. Chem. Spring, 2007 UMass-Amherst The lowest energy state is designated 4A 2g ("quartet A-two-g") and is the ground state. The "4" tells you the spin multiplicity (# unpaired electrons + 1), while the "A 2g" indicates the symmetry of the electronic state.
Answer to Write orbital diagrams for each of these ions. V5+,Cr3+,Ni2+,Fe3+ Determine if the ion is diamagnetic or paramagnetic. V 86%(14). Write orbital diagrams for each of these ions. A. V^5+ B. Cr^3+ C. Ni^2+ D. Fe^3+ E. Determine if the following ions are diamagnetic or paramagnetic.
November 7, 2021 - The purpose of introducing quantum numbers has been to show that similarities in the electron arrangement or electron configuration lead to the similarities and differences in the properties of …
Video explanation on the exceptions to electron configuration. There are two main exceptions to electron configuration: chromium and copper. Video explanation on both of these exceptions.
Limitations of MO Diagrams The problem: orbital energy diagrams ignore inter-electron repulsion, i.e., several states comprise the (t 2g)1(e g) 1 configuration,
Transcribed image text: Enter an orbital diagram for Cr Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. Not all targets will be filled. Reset Help 10 4p 11 11 (11 11 11 11 111111 GI DIDI GI GT GIGI 18 28 2p G2 3p 38 G2 Enter an orbital diagram for Ni2+ Drag the appropriate labels to their respective targets.
March 16, 2018 - How is the electron configuration of . Concepts and reason The concept of this question is that the filling of electrons in the shells and subshells of an atom takes place on the basis of Aufbau rule. It states that the subshells are arranged in increasing order of their n + l value where n ...
An atom is considered paramagnetic if even one orbital has a net spin. In contrast, paramagnetic and ferromagnetic materials are attracted by a magnetic field. V5+,Cr3+,Ni2+,Fe3+ Expert Answer 88% (33 ratings) Elementary Vanadium has electron configuration [Ar] 4s2 3d3 . Cd2+b.
The electronic configuration of Cr having atomic number of 24 is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 1 3d 5 which is half-filled d-orbital.. Cr3+ has 3 electrons removed from the outermost shell. Therefore, the electronic configuration comes out to be [Ar]3d3.
August 4, 2016 - The electron configuration for chromium is NOT 1s^2 2s^2 2p^6 3s^2 3p^6 3d^4 4s^2, but color(blue)(1s^2 2s^2 2p^6 3s^2 3p^6 3d^5 4s^1). Interestingly enough, Tungsten is more stable with an electron arrangement of [Xe]4f^14 5d^4 6s^2. Unfortunately, there is no easy way to explain these deviations ...
Solution · The electronic configuration of Cr(24) atom is: 1s22s22p63s23p64s13d5 which is half-filled d-orbital. Cr3+ has 3 electrons removed from the outermost ...19 Nov 2019
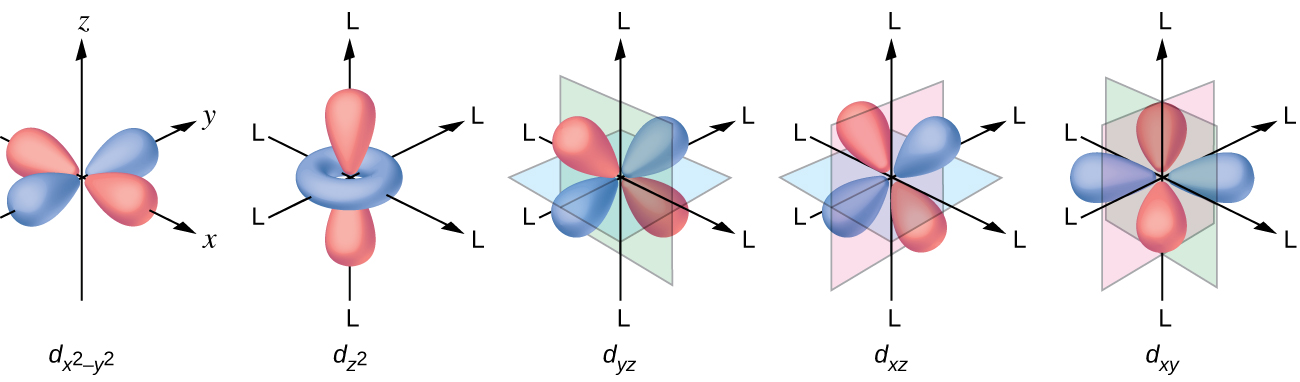
Orbital Splitting: The five d-orbitals are given the symbols dxy, dzx, dyz, dx 2-y 2 and dz 2. In a complex they are all differently aligned relative to the incoming charge. Depending on the geometry of the complex, some of the d-orbitals will point directly towards the ligands, while some will point between them.
Electrons are added to a subshell with the same value of the spin quantum number until each orbital in the subshell has at least one electron. Octahedral transition-metal ions with d 1, d 2, or d 3 configurations can therefore be described by the following diagrams. When we try to add a fourth electron, we are faced with a problem.
Write orbital diagrams for each ion and determine if the ion is diamagnetic or paramagnetic. a. V5+ b. Cr3+ c. Ni2+ d. Fe3+
The answer is [Ar] 4s1 3d5 Because half-filled subshells are somewhere stable, the atom prefers to have a half-filled 4s and half-filled 3d's than a full 4s ...
6 UCLES 2021 970142M21 (b) Heating of FeO results in the formation of Fe 3 O 4, as shown. reaction 1 4FeO → Fe + Fe 3 O 4 Each formula unit of Fe 3 O 4 contains one Fe2+ and two Fe3+ ions. (i) Show how reaction 1 can be described as a disproportionation reaction. [1] Fe 3 O 4(l) can be electrolysed using inert electrodes to form Fe. (ii) Write the half-equation for the reaction that occurs ...
However, even though the 5s orbital is lower in energy than the 4d orbital, the electrons in the 4d orbitals shield the electron in the 5s orbitals. can be accommodated in the metal d orbitals. • d0 ions d3 ions - V2+, Ta2+, Cr3+, Mo3+, Mn4+, etc. . σ-ML4 Tetrahedral MO Diagram e. Answer to Write orbital diagram for Mo3+.
After the 4s is full we put the remaining six electrons in the 3d orbital and end with 3d6. Therefore the Iron electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6. Note that when writing the electron configuration for an atom like Fe, the 3d is usually written before the 4s. Both of the configurations have the correct numbers of ...















0 Response to "37 cr3+ orbital diagram"
Post a Comment