36 diagram of exothermic reaction
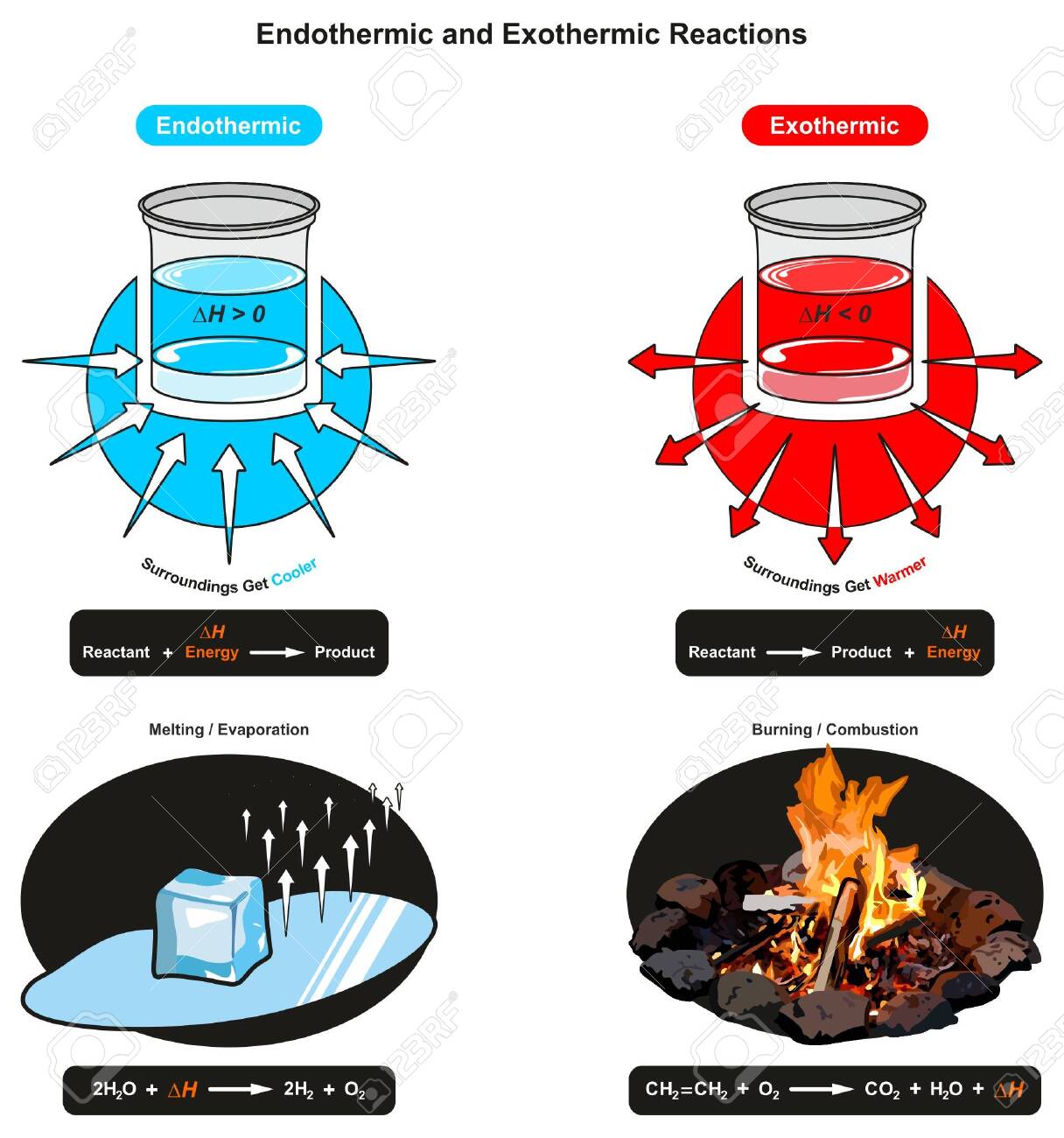
Exothermic Reaction: When methane gas is combusted, heat is released, making the reaction exothermic. Specifically, the combustion of \(1 \: \text{mol}\) of methane releases 890.4 kilojoules of heat energy. This information can be shown as part of the balanced equation in two ways. First, the amount of heat released can be written in the product side of the reaction. Another way is to write ... An exothermic process releases heat, causing the temperature of the immediate surroundings to rise. An endothermic process absorbs heat and cools the surroundings.". Based on the above definition, let's pick a few examples from our daily lives and categorize them as endothermic or exothermic.
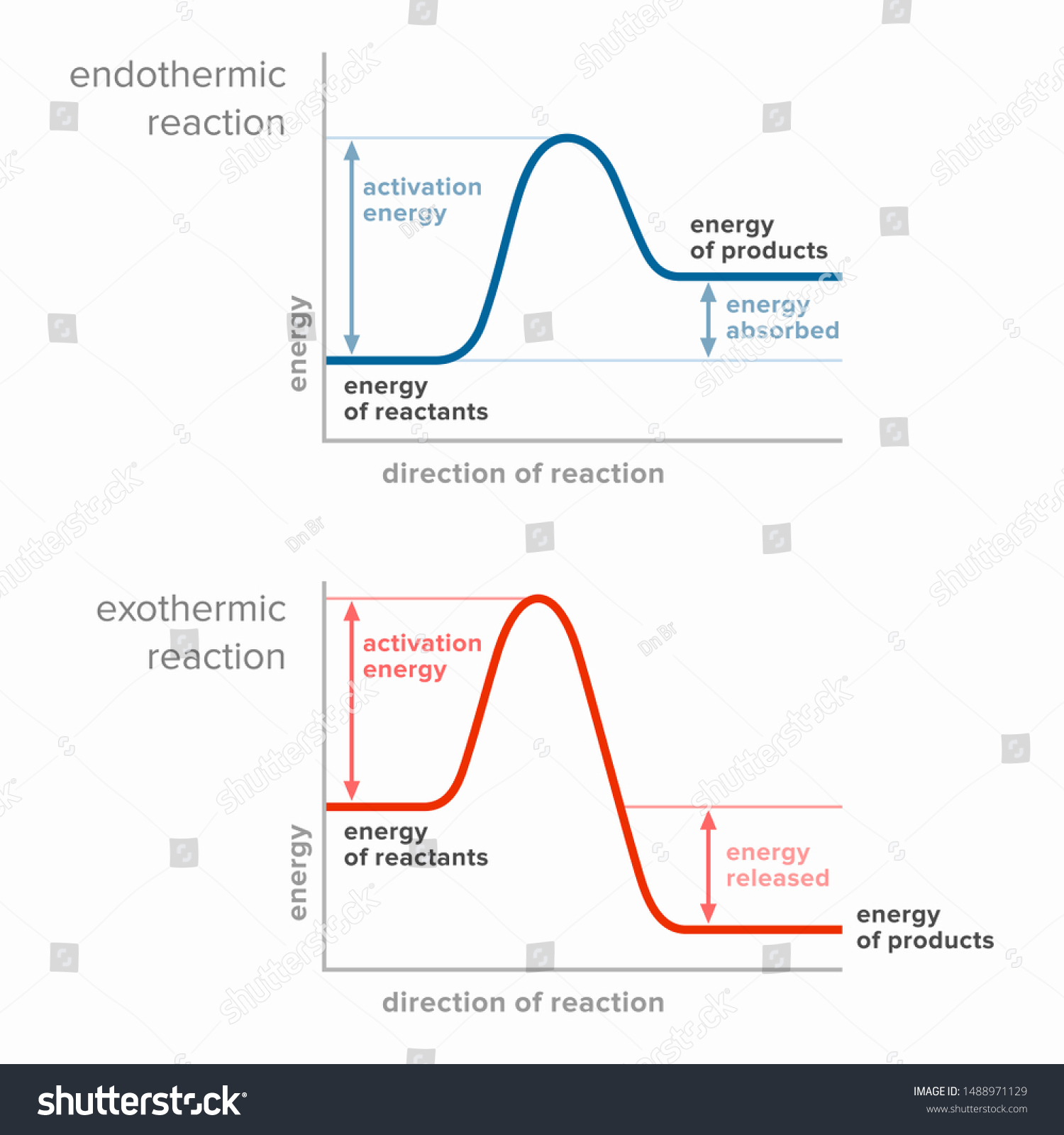
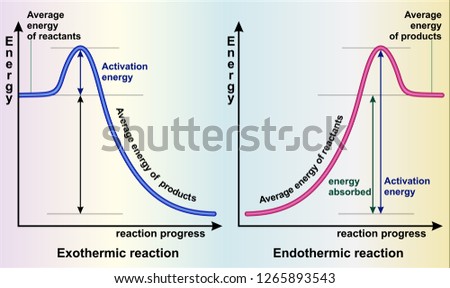
Endothermic Reaction Energy Level Diagram: Endothermic reactions are depicted in a basic energy level diagram below. The activation energy is the amount of energy that must be delivered to the reactants for them to break through the energy barrier and react. In an endothermic reaction, the result has higher potential energy than the reactants.

Diagram of exothermic reaction
Exothermic and Endothermic Reactions - Energy Level DiagramForm 5 Chemistry Chapter 4 ThermochemistryThis video is created by http://www.onlinetuition.com.my... 28 Aug 2021 — From an energy level diagram, we can determine the following: 1. Is the reaction endothermic or exothermic? 2. Does the product have more energy ... Transcribed image text: The reaction energy diagrams for an endothermic and an exothermic reaction are shown below. Observe the graphs, and classify the following properties of exothermic and endothermic reactions. Energy of reactants Activation energy Potential energy Potential energy Change in Energy of products Change in potential energy Activation ghergy Energy of reactants potential ...
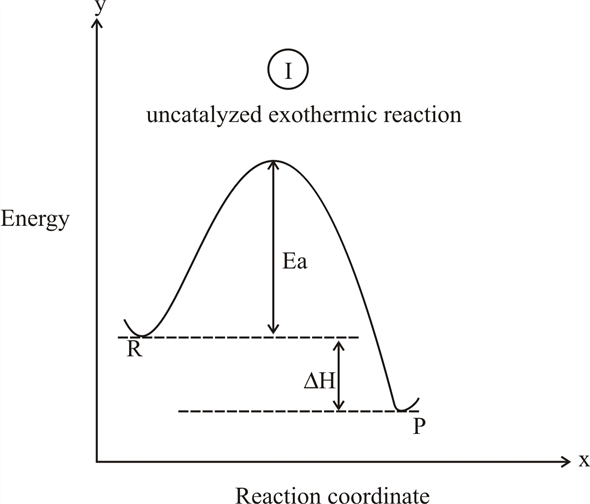
Diagram of exothermic reaction. Draw an energy diagram for a two step, overall exothermic process. Indicate locations of all energies, transition states, and intermediate states on this diagram. Draw an energy diagram for a two-step reaction that is exothermic overall, and consists of a fast but endothermic first step, and a slow but exothermic second step. Indicate DG rxn , as well as DG 1 * and DG 2 * for the first and second activation energies, respectively. The reaction coordinate diagram for the ozone photolysis reaction is a little different from those above because this is an endothermic reaction. Together, the products O 2 and atomic O, have a higher energy than the reactant O 3 and energy must be added to the system for this reaction. An exothermic reaction is the release of thermal energy (-ΔH) as it flows out of the system. Thermal energy is negative because energy is being released and the initial potential energy of reactants is more than the energy released from products. Let's explore the exothermic reactions occurring around us: 1.
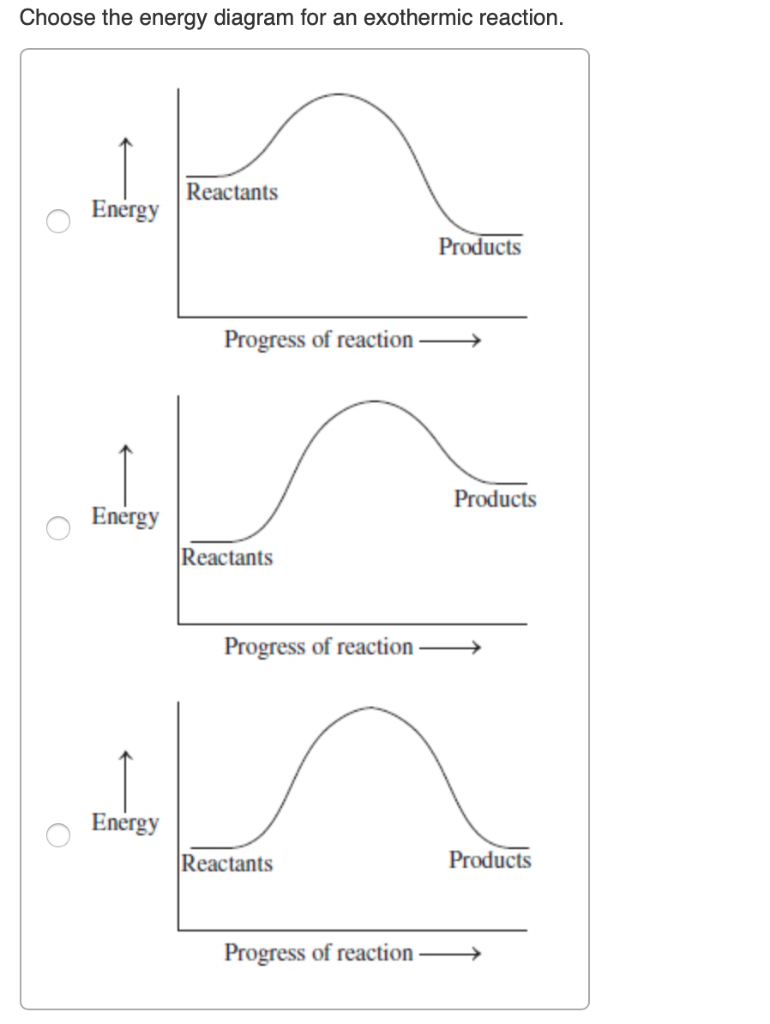
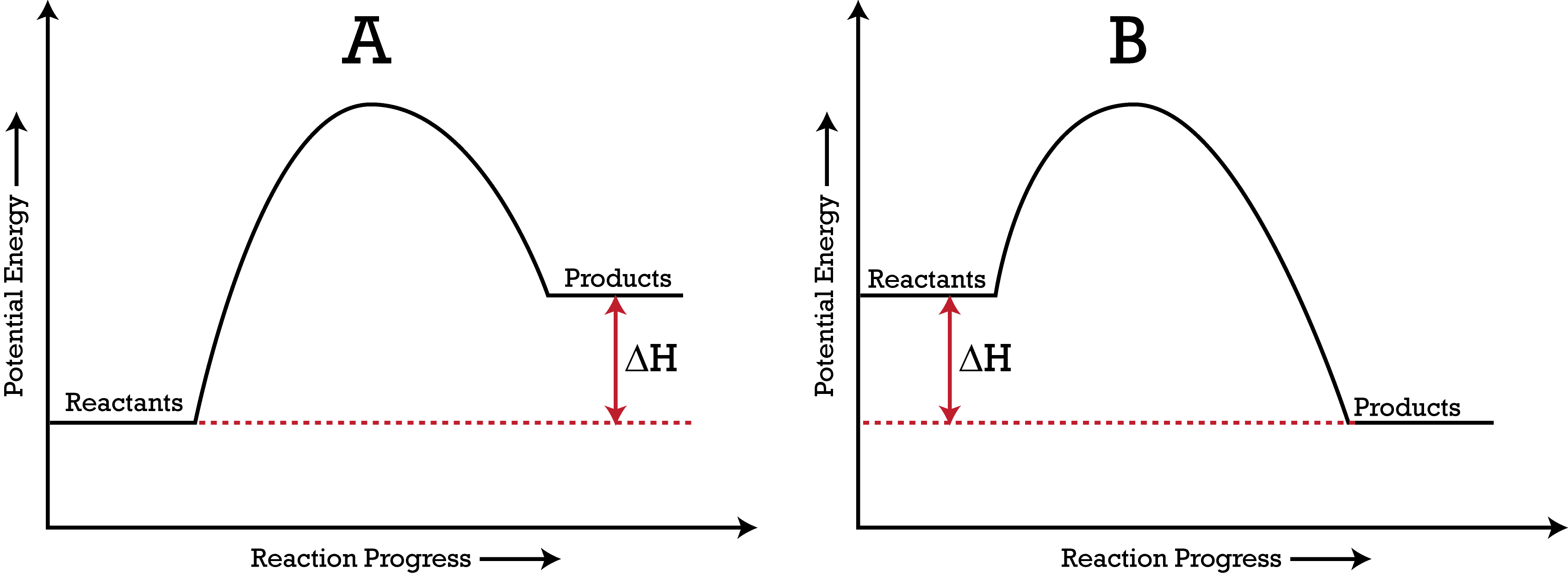
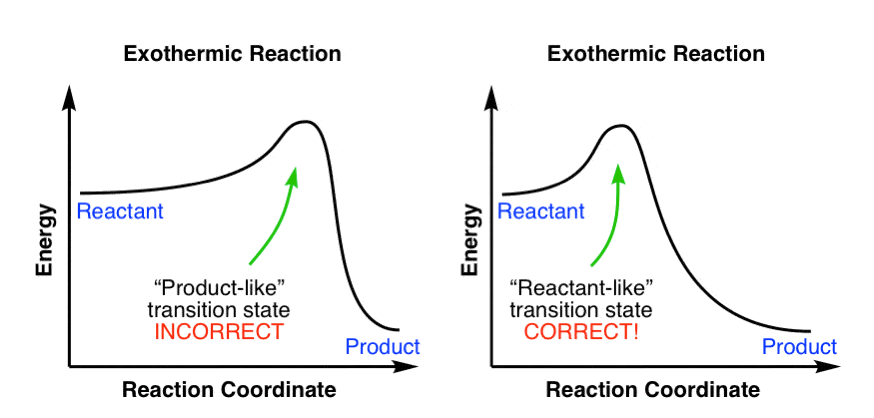
You can start with a generic potential energy diagram for an exothermic reaction.. A reaction is defined as exothermic if you put in less energy to break the bonds of the reactants - the is the activation energy - than it is released when the products are formed.. So, the activation energy is the minimum amount of energy required for a reaction to take place. Which potential energy diagram represents an exothermic reaction? Potential Energy Potential Energy non Reaction coordinate A) Reaction coordinate B) Reaction coordinate C) Reaction coordinate D) Select an answer and submit. For keyboard navigation, use the up/down arrow keys to select an answer. a a b b с c d d Which potential energy diagram ... Figure shows the energy level diagram for the reaction between methane and oxygen. Based on Figure, the following information can be obtained. (a) The reaction between methane and oxygen to form carbon dioxide and water is an exothermic reaction. (b) During the reaction, the temperature of the mixture increases. Exothermic and endothermic reactions. When a chemical reaction occurs, energy is transferred to or from the surroundings. There is usually a temperature change.
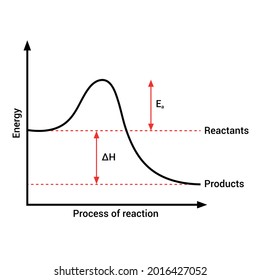
1. Identify the general shape of the energy diagram Energy should conserve for any chemical reaction. The reaction in question is exothermic (releases heat) hence its products shall have chemical potential energies lower than that of its reactants- some of the potential energies have been converted to thermal energy during the reaction process. An exothermic reaction is a chemical reaction that releases heat and has a negative enthalpy (-ΔH) and positive entropy (+ΔS).. These reactions are energetically favorable and often occur spontaneously, but sometimes you need a little extra energy to get them started. 9 Jul 2021 — Recall that the enthalpy change (ΔH) is positive for an endothermic reaction and negative for an exothermic reaction. This can be seen in the ... Exothermic Reactions is the flow of the net transfer of heat energy during the reaction is from the medium into its surroundings. In exothermic reactions, the reactants always possess more energy than the products and hence are less stable. For this reason, the exothermic reactions require very less amount of activation energy to initiate the reaction.
Start studying Chemistry H, Venn Diagram {Exothermic vs. Endothermic} :). Learn vocabulary, terms, and more with flashcards, games, and other study tools.
Energy Diagrams. Exothermic Reactions. Endothermic Reactions. Example. 6.3 Kinetic Energy, Heat Transfer, and Thermal Equilibrium. 6.4 Heat Capacity and Coffee-Cup Calorimetry. 6.5 Phase Changes and Energy. 6.6 Introduction to Enthalpy of Reaction. 6.7 Bond Enthalpy and Bond Dissociation Energy.
Energy Diagrams Both endothermic and exothermic reactions can be shown on energy diagrams. A way to tell if a diagram is endothermic or exothermic reaction is to look at the start and end of the graph. If the end part of the graph is more than the start, it is an endothermic reaction meaning it absorbed energy.
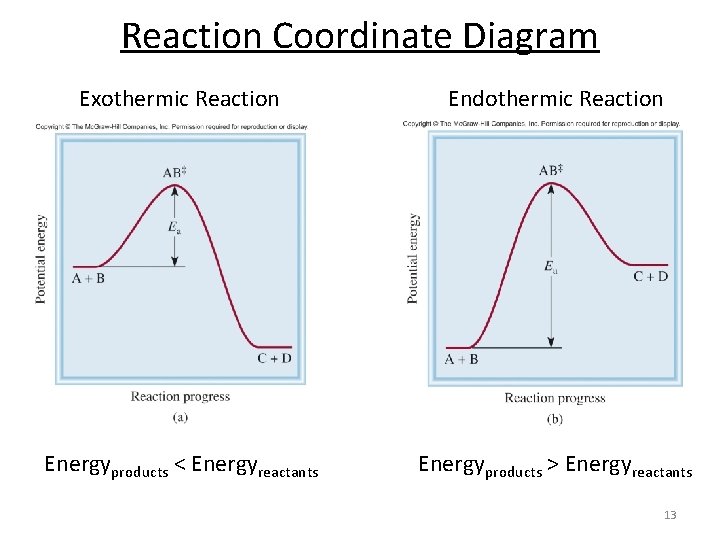
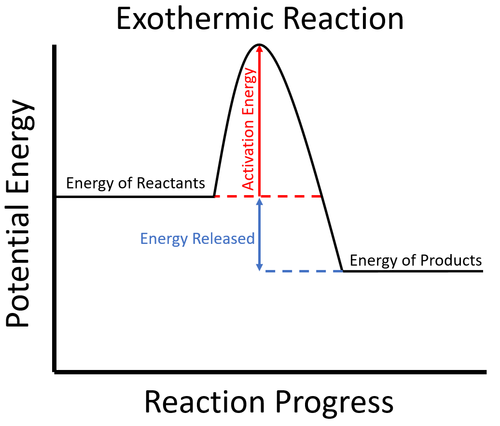
Energy level diagrams for exothermic reactions In an exothermic reaction, reactants have more energy than the products . The difference between these two energy levels is the energy released to the surroundings, shown as a vertical drop from a higher to a lower level. Because the reactants have more energy than the products they are less stable. 6 | P a g e Usually some extra energy is needed ...
Energy Profile for Exothermic Reactions. The synthesis of ammonia gas (NH 3 (g)) from nitrogen gas (N 2 (g)) and hydrogen gas (H 2 (g)) is an exothermic reaction. 92.4 kJ mol -1 (of N 2 (g)) is released. Energy (heat) is a product of the reaction: N 2 (g) + 3H 2 (g) → 2NH 3 (g) + 92.4 kJ mol -1. In order for energy to be conserved during the ...
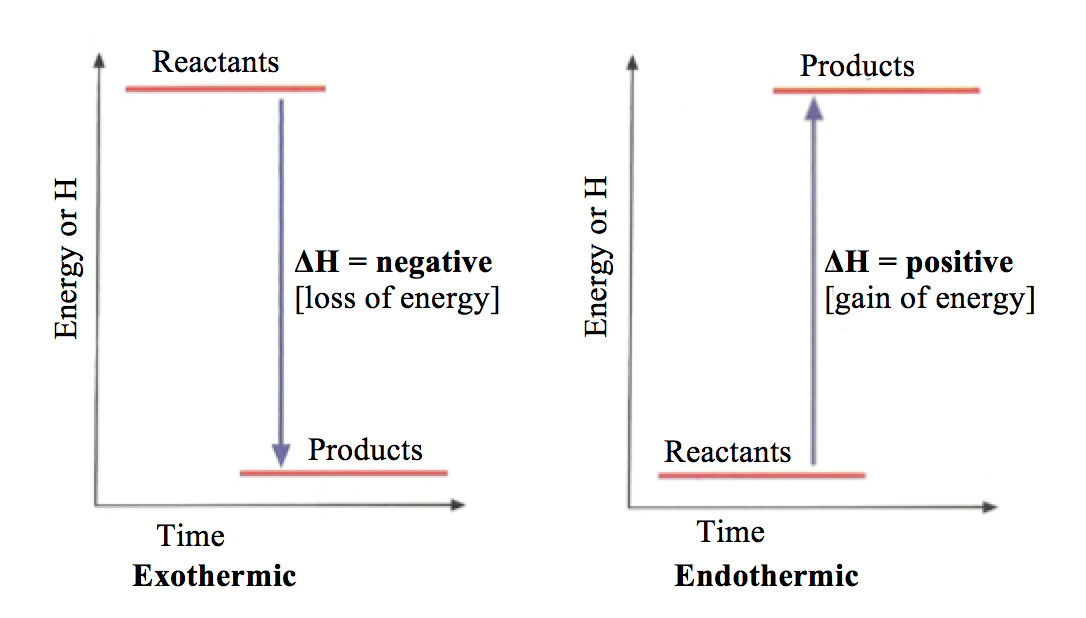
An exothermic reaction is a reaction in which energy is released in the form of light or heat. Thus in an exothermic reaction, energy is transferred into the surroundings rather than taking energy from the surroundings as in an endothermic reaction. In an exothermic reaction, change in enthalpy ( ΔH) will be negative.
Energy Diagrams. Exothermic Reactions. Endothermic Reactions. Example. 6.3 Kinetic Energy, Heat Transfer, and Thermal Equilibrium. 6.4 Heat Capacity and Coffee-Cup Calorimetry. 6.5 Phase Changes and Energy. 6.6 Introduction to Enthalpy of Reaction. 6.7 Bond Enthalpy and Bond Dissociation Energy.
Diagram of endothermic and exothermic reactions. Learn with flashcards, games, and more — for free.
Reaction Coordinate Diagrams Let's consider a general reaction where a reactant or set of reactants, A, is transformed into a product or set of products, B. The diagram below is called a reaction coordinate diagram. It shows how the energy of the system changes during a chemical reaction. In this example, B is at a lower total energy than A. This is an exothermic reaction (heat is given off ...
The enthalpy diagram for exothermic and endothermic reactions is shown below. Explanation : Endothermic reaction : It is defined as the chemical reaction in which the energy is absorbed from the surrounding. In the endothermic reaction, the energy of reactant are less than the energy of product.
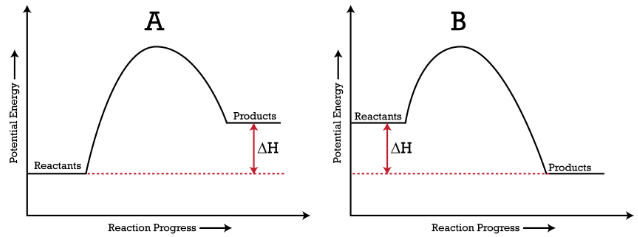
a) Draw a diagram of the energy profile for this reaction. Label the diagram. b) State whether the reaction is endothermic or exothermic. c) Calculate the energy difference between the reactants and the products. d) Deduce the sign of the enthalpy change. e) Identify with a reason, which is more stable, the reactants of products. 8. (N04/S/2)
Key Facts. Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. A good example of an endothermic reaction is photosynthesis. Combustion is an example of an exothermic reaction. The categorization of a reaction as endo- or exothermic depends on the net heat transfer.
Exothermic Diagram. Energy released in bond making. Activation Energy. Energy used in bond. breaking. Exothermic - More energy is released when the products where formed than energy was used to break bonds in the reactants. Therefore, a net release of energy.
An energy level diagram. shows whether a reaction is exothermic. or endothermic. It shows the energy in the reactants and products , and the difference in energy between them. Exothermic reaction
Diagram for Endothermic Reaction. Burning methane was an exothermic reaction, now how do we draw the energy diagram for an endothermic reaction? An example of this is when we dissolve ammonium ...
ENERGY IS A REACTANT, SO THE REACTION IS ENDOTHERMIC AND ΔH IS POSITIVE! Page 5. EQUATIONS &. ENERGY DIAGRAMS. • WE CAN USE AN ENERGY DIAGRAM ...11 pages
This chemistry video tutorial focuses on potential energy diagrams for endothermic and exothermic reactions. It also shows the effect of a catalyst on the f...
Transcribed image text: The reaction energy diagrams for an endothermic and an exothermic reaction are shown below. Observe the graphs, and classify the following properties of exothermic and endothermic reactions. Energy of reactants Activation energy Potential energy Potential energy Change in Energy of products Change in potential energy Activation ghergy Energy of reactants potential ...
28 Aug 2021 — From an energy level diagram, we can determine the following: 1. Is the reaction endothermic or exothermic? 2. Does the product have more energy ...
Exothermic and Endothermic Reactions - Energy Level DiagramForm 5 Chemistry Chapter 4 ThermochemistryThis video is created by http://www.onlinetuition.com.my...


















0 Response to "36 diagram of exothermic reaction"
Post a Comment