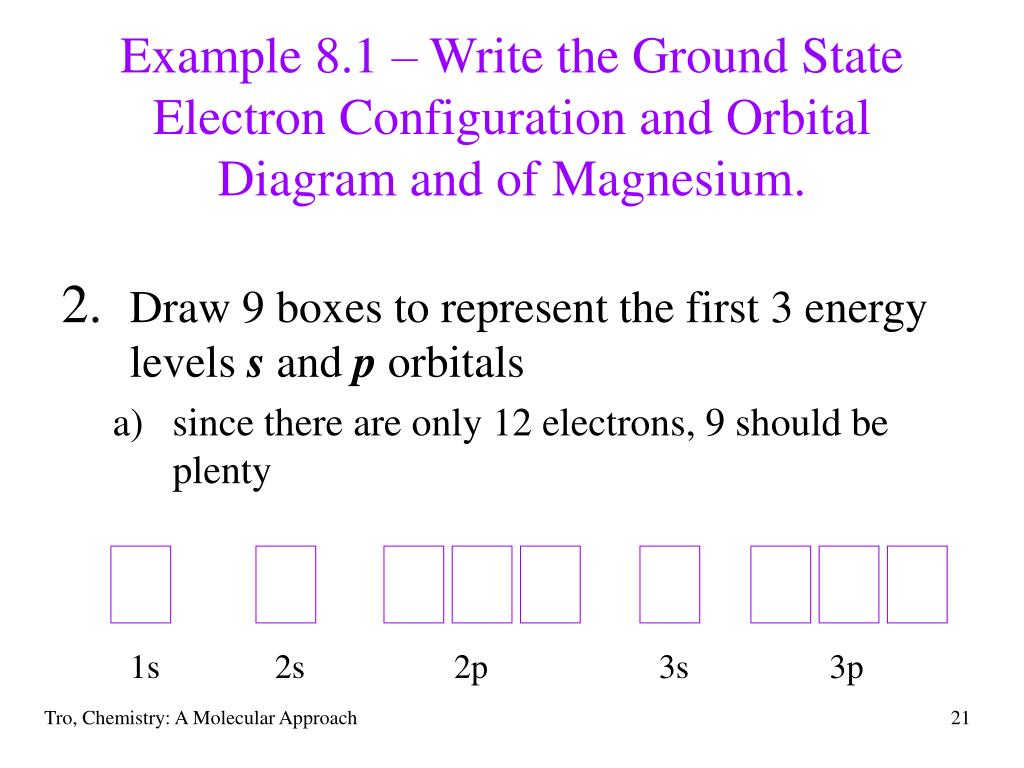
38 orbital diagram for mg
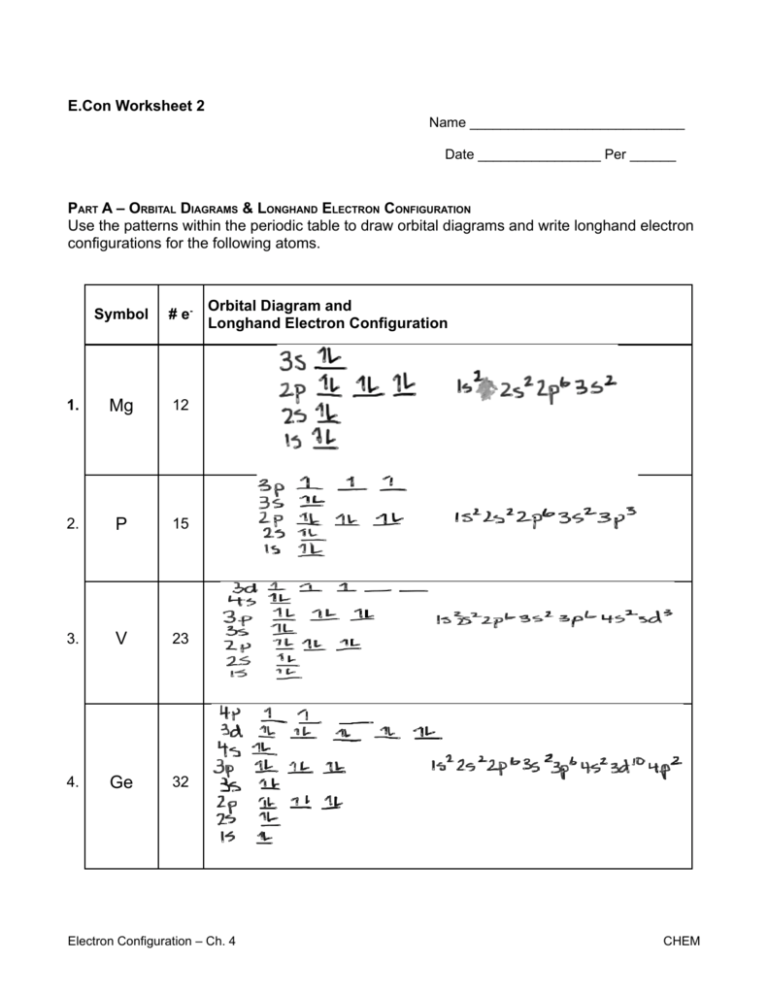
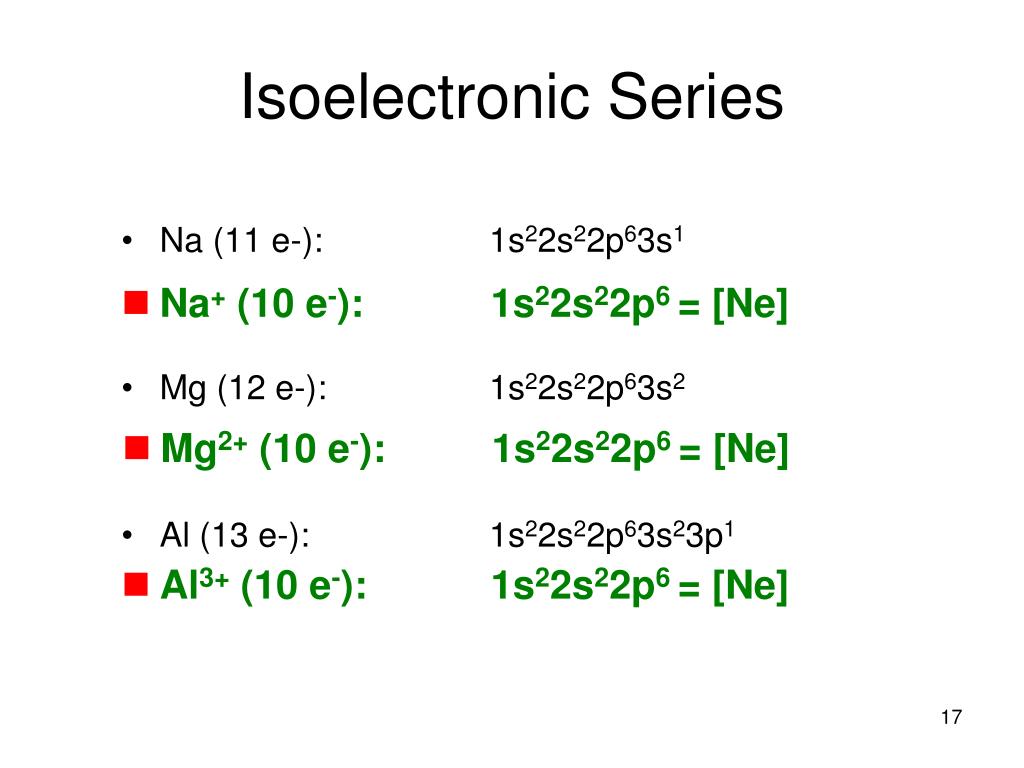
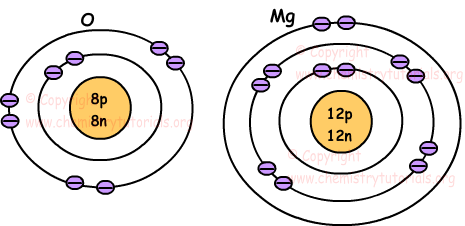
Magnesium (Mg) has an atomic mass of 12. ... Electron Configuration, [Ne] 3s2. 1s2 2s2 2p6 3s2. Orbital Diagram ... Lewis Dot Diagram of Magnesium (Mg). 2:15Magnesium has 2 electrons in its first shell, 8 in its second and 2 in its third.Check me out: http://www ...24 Jul 2019 · Uploaded by chemistNATE
The fourth orbital in this subshell lies along the X and Y axes and is called the 3d x 2-y 2 orbital. Most of the space occupied by the fifth orbital lies along the Z axis and this orbital is called the 3d z 2 orbital. The number of orbitals in a shell is the square of the principal quantum number: 1 2 = 1, 2 2 = 4, 3 2 = 9.

Orbital diagram for mg
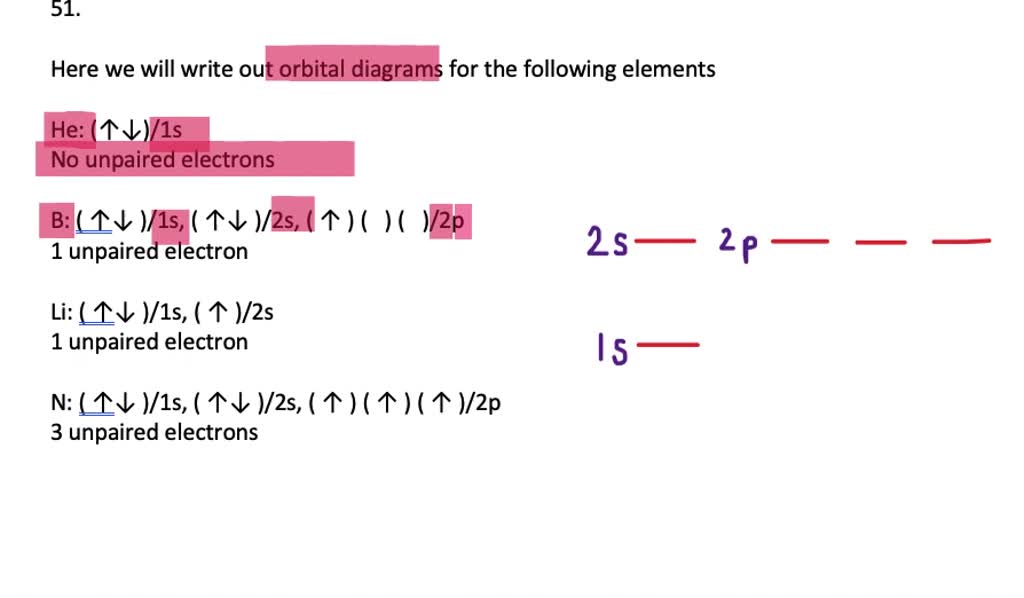
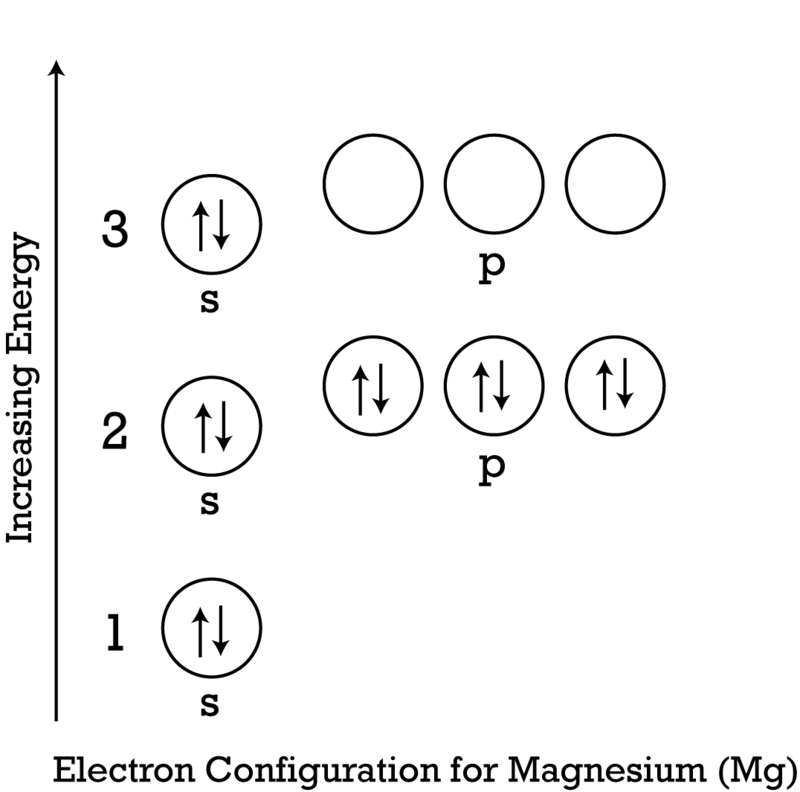
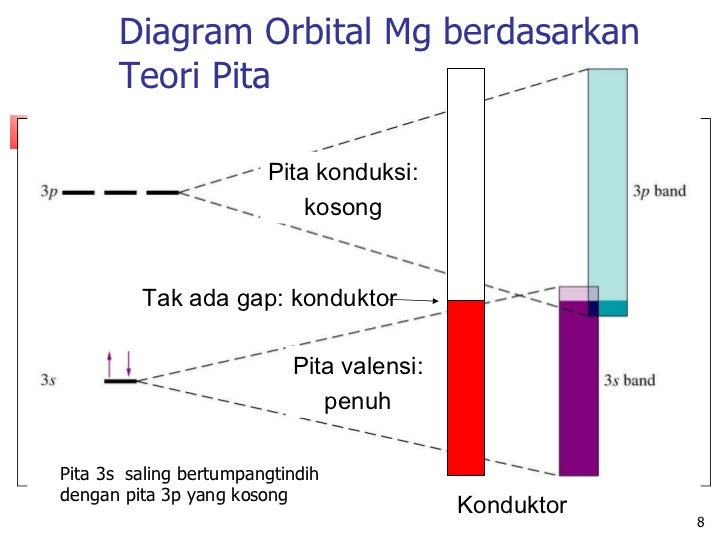
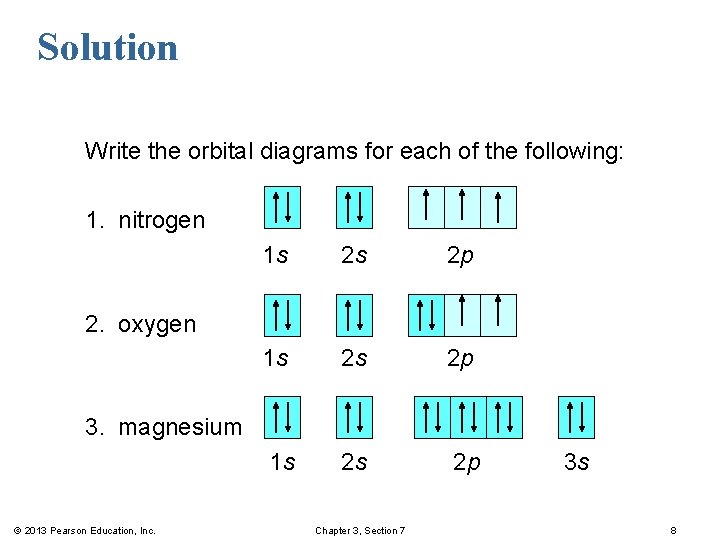
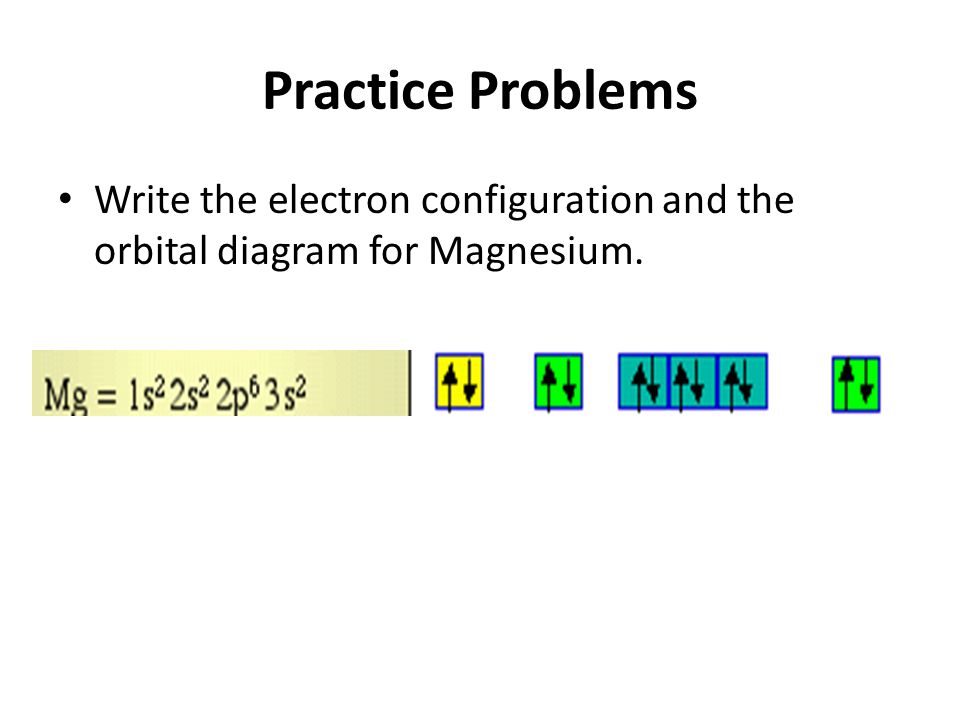
In picture 1 we show the molecular orbital structure of F2. In picture 2 we show the overlapping p orbitals, which form the bond between the two fl uorine atoms, in red and green gradients. The dashed lines show the remaining p orbitals which do not take part in the bonding. σ z y x σ* x y z Construct the molecular orbital diagram for ... Since 1s can only hold two electrons the next 2 electrons for magnesium go in the 2s orbital. The nex six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the remaining two electrons in the 3s. Therefore the Magnesium electron configuration will be 1s 2 2s 2 2p 6 3s 2. We’re being asked to construct the orbital diagram for Mg. For that, we first need to determine the electron configuration of Mg. Recall that for a neutral element, Atomic number = # of protons = # of electrons . The atomic number of Mg is 12 and since it’s a neutral element, this means Mg has 12 electrons. 82% (287 ratings)
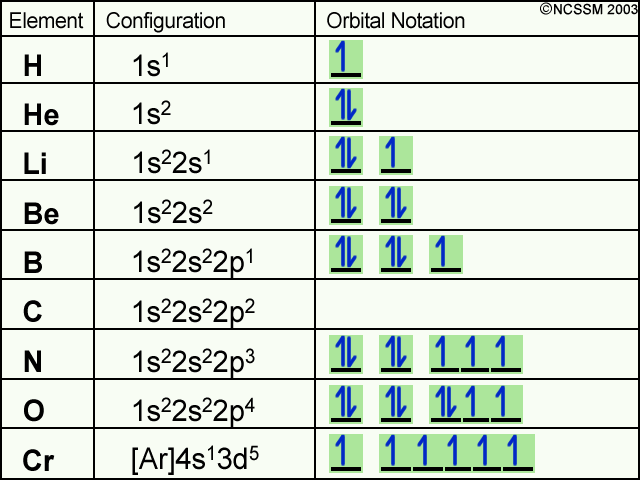
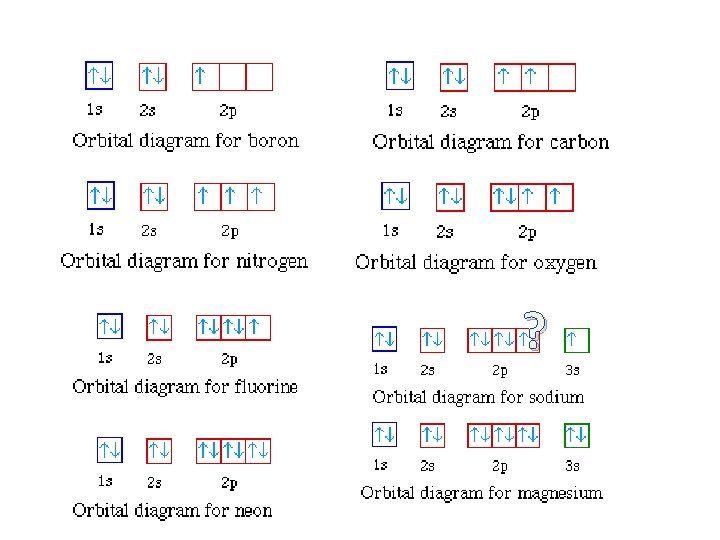
Orbital diagram for mg. MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H–F nb σ σ* Energy H –13.6 eV 1s F –18.6 eV –40.2 eV 2s 2p So H–F has one σ bond and three lone electron pairs on fluorine 1:36A step-by-step description of how to write the electron configuration for Magnesium (Mg). In order to write the ...18 Nov 2013 · Uploaded by Wayne Breslyn 26 Jan 2021 — 1s2 2s2 2p6 3s2 is the Ground-state Electron Configuration of the Mg. ... Many other valence electrons of the element have been available here:. Orbital diagram of Lithium (Li) 4: Orbital diagram of Beryllium (Be) 5: Orbital diagram of Boron (B) 6: Orbital diagram of Carbon (C) 7: Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine (F) 10: Orbital diagram of Neon (Ne) 11: Orbital diagram of Sodium (Na) 12: Orbital diagram of Magnesium (Mg) 13 ...
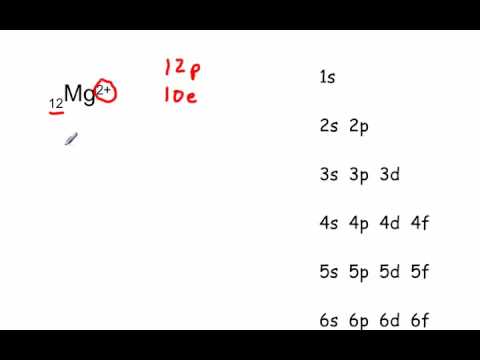
Answer: This just shows energy levels so let's take this a step further. Atomic Electron Configurations And I'm not having any luck but if you go to this site, you should be about to see what the 1s, 2s, 2px, 2py, 2pz, and 3s orbitals look like together. Jmol orbital structures If not, see what... The orbital notation of magnesium is 1S2 2S2 2P6 3S2. Each orbital is indicated by a line and can contain two electrons that are drawn as up and down arrows. What is the orbital diagram for Magnesium? 2:27In this video we will write the electron configuration for Mg 2+, the Magnesium ion. We'll also look at why ...19 Jun 2019 · Uploaded by Wayne Breslyn 12:12It explains how to write the orbital diagram notation (with arrows) of an element given its atomic number and by ...26 Sep 2017 · Uploaded by The Organic Chemistry Tutor
4. Spin Quantum Number (ms): m s = +½ or -½. Specifies the orientation of the spin axis of an electron. An electron can spin in only one of two directions (sometimes called up and down). The Pauli exclusion principle (Wolfgang Pauli, Nobel Prize 1945) states thatno two electrons in the same atom can have identical values for all four of their quantum numbers. 11:05This chemistry video tutorial explains how to find the electron configuration of Ions such as Mg2+, P3-, Fe2+ ...21 Jun 2020 · Uploaded by The Organic Chemistry Tutor The four quantum numbers determine the state of the electron, and are. ms is the spin of the electrons inside the orbital ( − 1 2 or + 1 2 ). The electronic configuration for magnesium is 1s22s22p63s2. The outermost electron is one in the 3s orbital, which means that n = 3. It also means that l = 0, since s is basically shorthand for the ... We’re being asked to construct the orbital diagram for Mg. For that, we first need to determine the electron configuration of Mg. Recall that for a neutral element, Atomic number = # of protons = # of electrons . The atomic number of Mg is 12 and since it’s a neutral element, this means Mg has 12 electrons. 82% (287 ratings)
Since 1s can only hold two electrons the next 2 electrons for magnesium go in the 2s orbital. The nex six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the remaining two electrons in the 3s. Therefore the Magnesium electron configuration will be 1s 2 2s 2 2p 6 3s 2.
In picture 1 we show the molecular orbital structure of F2. In picture 2 we show the overlapping p orbitals, which form the bond between the two fl uorine atoms, in red and green gradients. The dashed lines show the remaining p orbitals which do not take part in the bonding. σ z y x σ* x y z Construct the molecular orbital diagram for ...
Solved A Write The Electron Configuration Draw The Orbital Diagram Determine The Distinguishing Electron And Determine The 4 Quantum Numbers For The Distinguishing Electron Of The Element Magnesium Mg Write Electron Configurations As

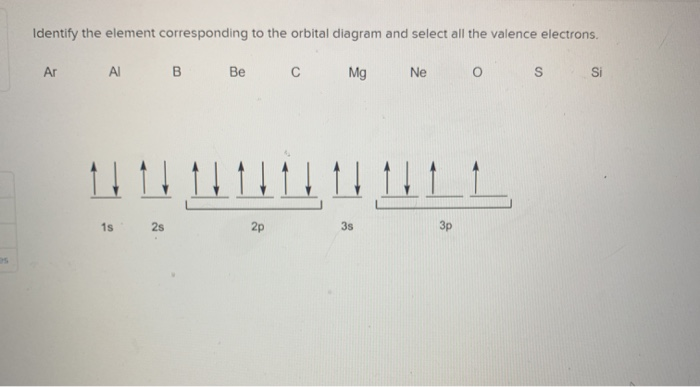
Solved Identify The Element Corresponding To The Orbital Diagram And Select All The Valence Electrons Ca Ga Na Mg Lllvw4ll
Solved Draw An Energy Level Diagram For Bromine Write The Short Electron Configuration For Magnesium Write The Long Form Electron Configuration Course Hero
Solved Construct An Orbital Diagram To Show The Electron Configuration For A Neutral Magnesium Atom Mg Mg Drag The Appropriate Labels To Their Res Course Hero







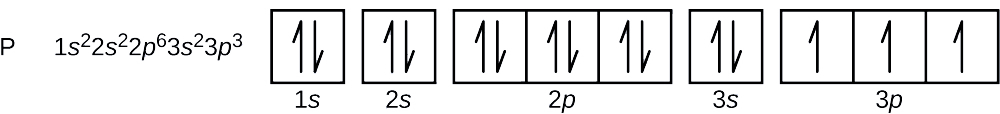








0 Response to "38 orbital diagram for mg"
Post a Comment