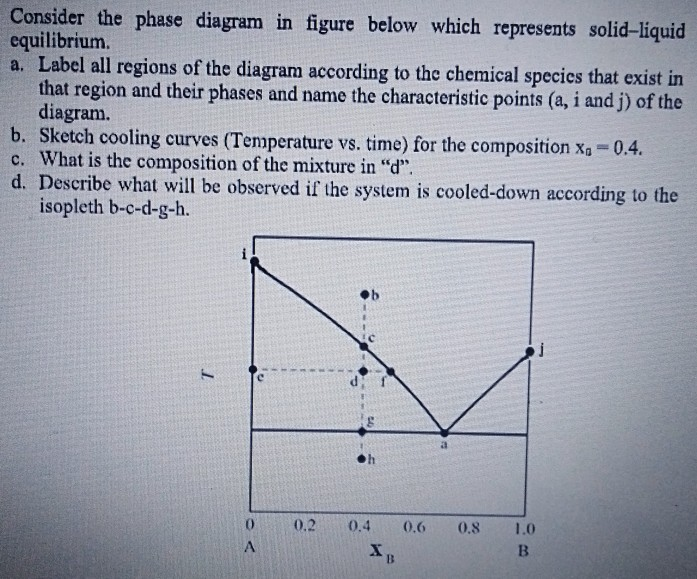
37 solid liquid phase diagram
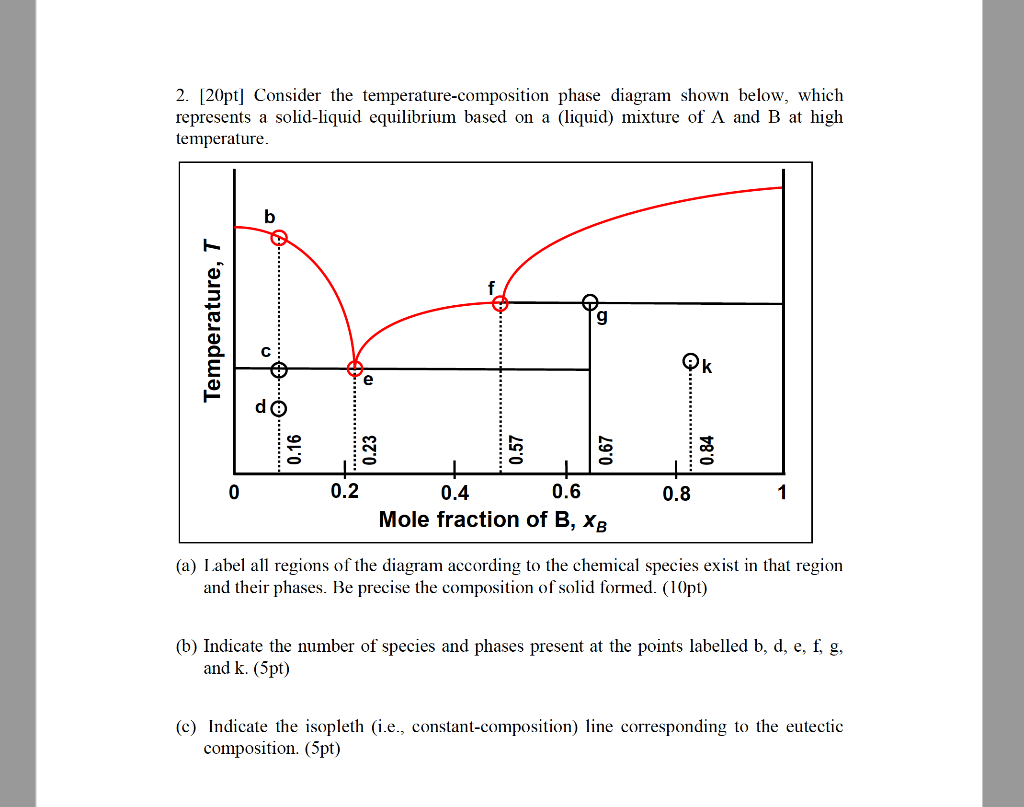
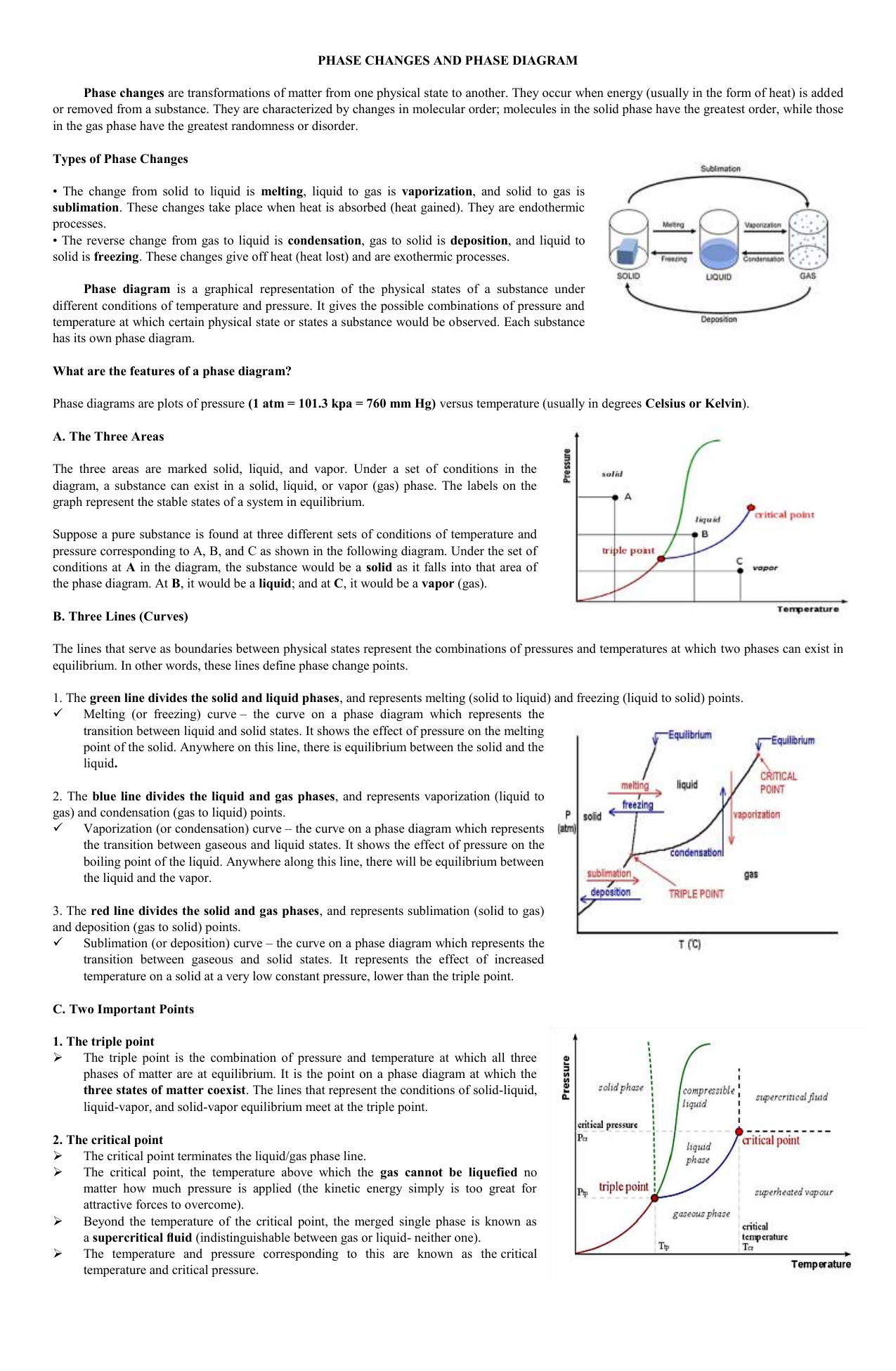
•The phase diagram plots relative concentrations of A and B along the X-axis, and temperature along the Y-axis. The eutectic point is the point where the liquid phase borders directly on the solid α + β phase; it represents the minimum melting temperature of any possible A B alloy. by T Htira · 2016 · Cited by 13 — The solid–liquid phase equilibrium implies the equality between the chemical potential of the water in the solid and liquid phases. Since the ...
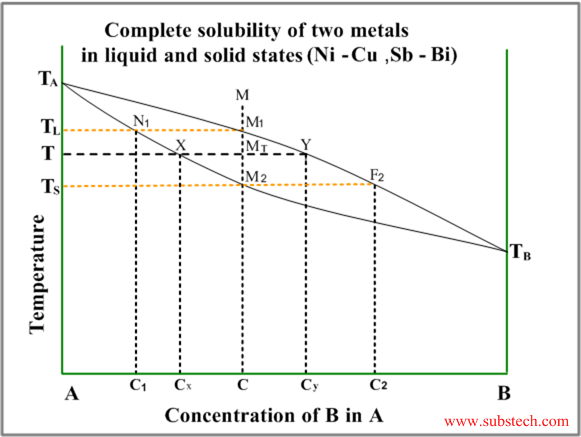
The solid solution phase diagram explains the behavior of chemical solid solution series, such as the transition from high temperature, calcium-rich plagioclase to low temperature sodium-rich plagioclase, or the transition from high temperature magnesium-rich to low temperature iron-rich crystals in ferromagnesium minerals (e.g. olivine, pyroxene).
Solid liquid phase diagram
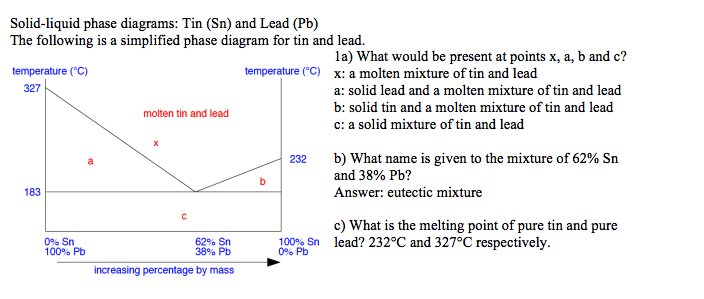
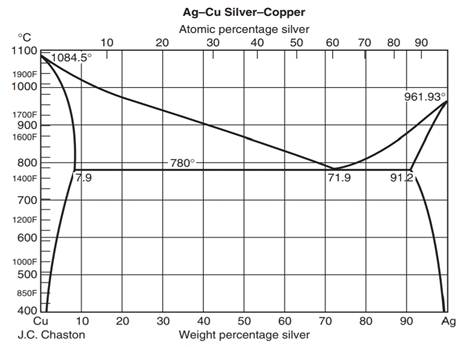
2.7 Liquid solid phase diagrams · Crystallization of B releases latent heat slope of cooling curve is reduced · When reaching the eutectic line cooling stops by ... by C Liu — The phase diagram belongs to a binary simple system with one eutectic point, and the content of C18-OH at eutectic point is 0.4 in mass fraction ... 12.1.3 Solid-Liquid Equilibrium Phase Diagrams ... Solid-liquid equilibrium data are obtained experimentally by cooling a liquid mixture of known composition and ...
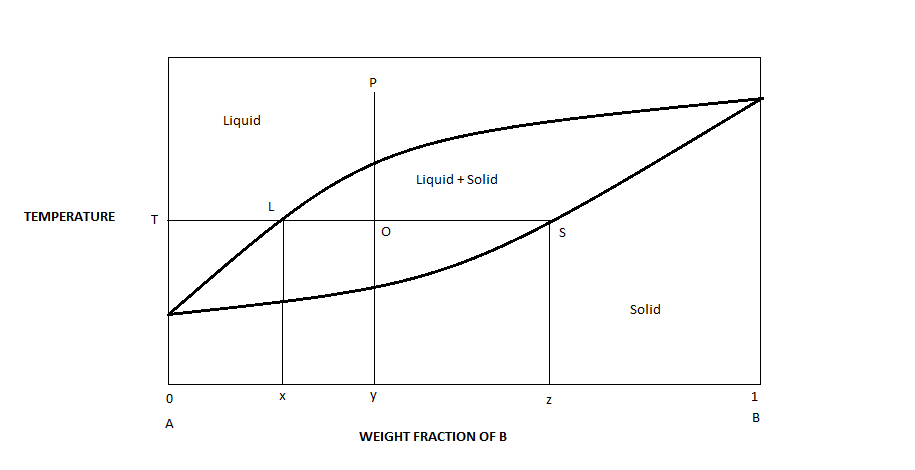
Solid liquid phase diagram. MSE 2090: Introduction to Materials Science Chapter 9, Phase Diagrams 11 Isomorphous system - complete solid solubility of the two components (both in the liquid and solid phases). Binary Isomorphous Systems (I) Three phase region can be identified on the phase diagram: Liquid (L) , solid + liquid (α +L), solid (α ) Look at the lines on the phase diagram. If you go to a line and then increase pressure you move to a new phase, the denser phase. Edit: Add the below. Go to the dotted green line. Now, go straight up (increase in P). What happened? You went from solid and liquid being in equilibrium to just liqui. The liquid is more dense than the solid. For most substances, the solid–liquid phase boundary (or fusion curve) in the phase diagram has a positive slope so that the melting point increases with ...Types · 2-dimensional diagrams · 3-dimensional diagrams · Binary mixtures The system enters the two phase region labeled 'liquid + B'. Pure solid B begins to come out of solution and the remaining liquid becomes richer in A. (2) a 2 ® a 3. More of the solid forms, and the relative amounts of the solid and liquid (which are in equilibrium) are given by the lever rule.
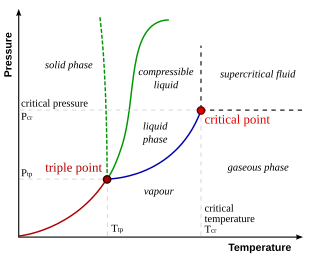
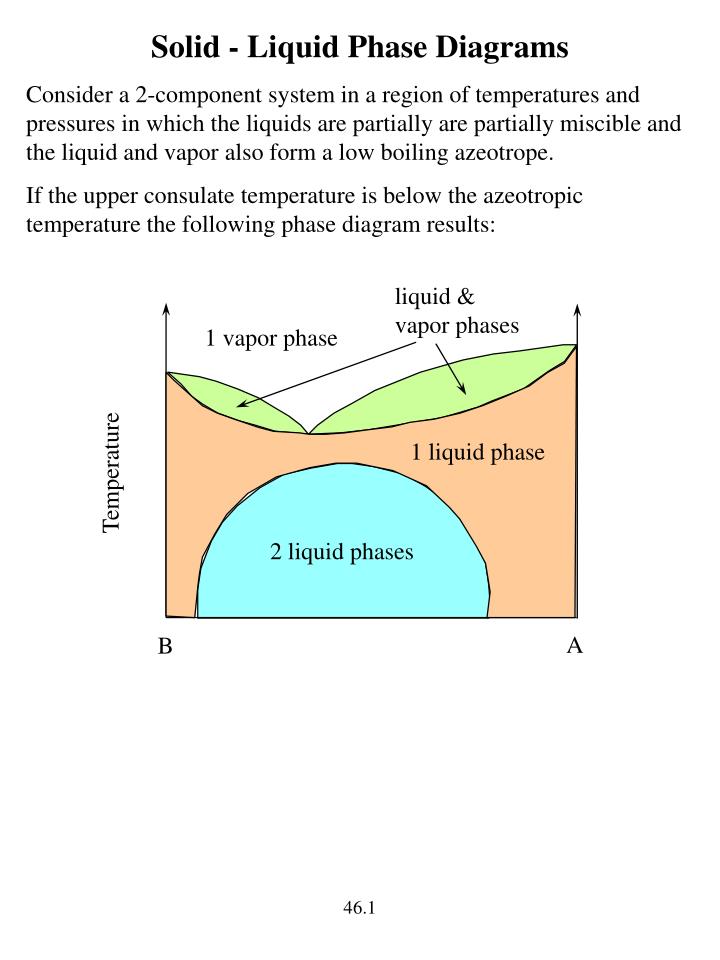
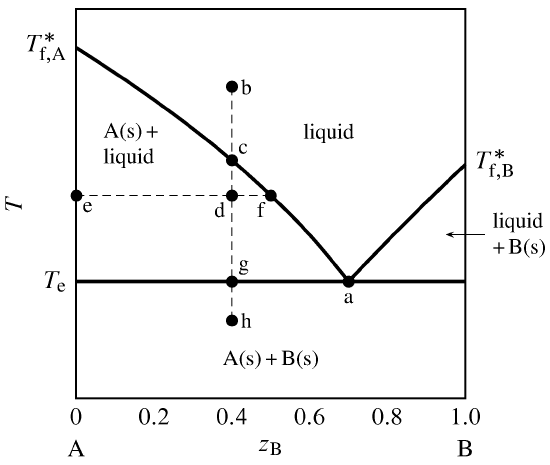
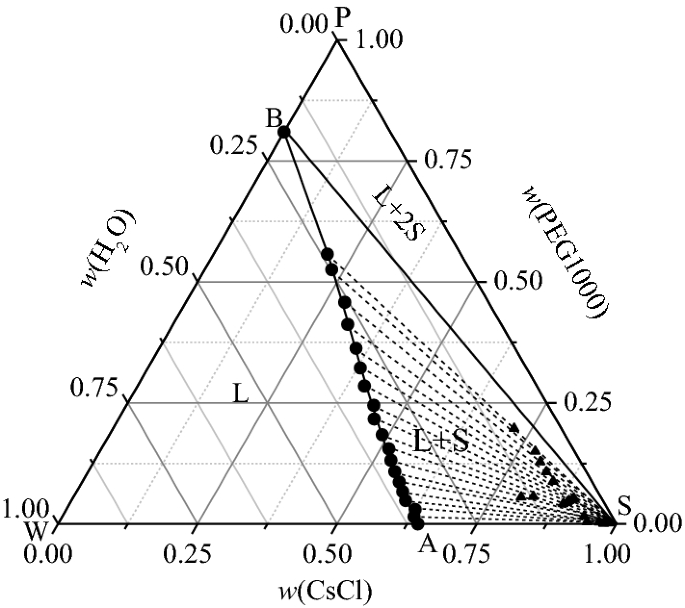
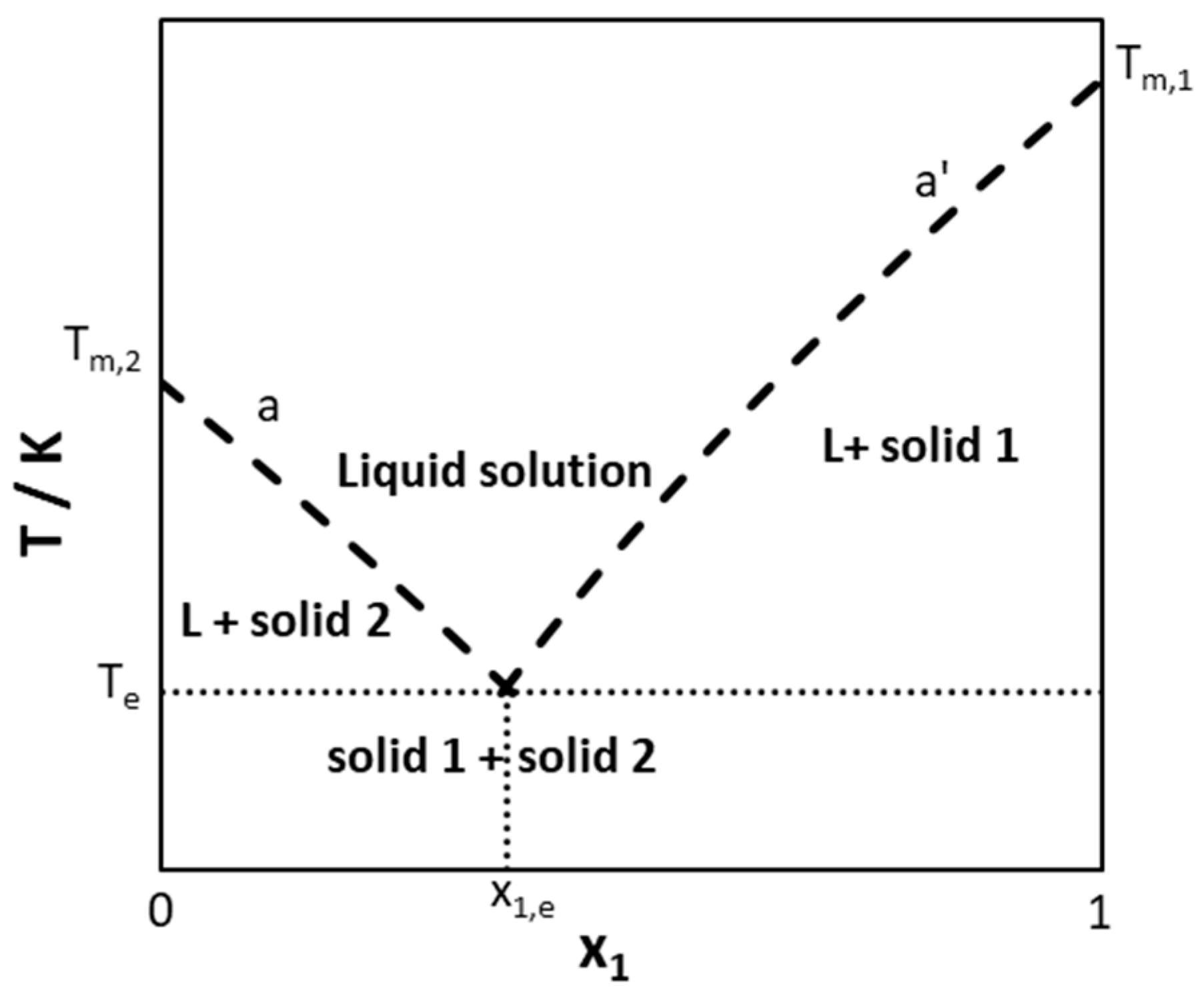
Binary Solid-Liquid Phase Diagram. Author: J. M. McCormick. Last Update: August 11, 2009. Introduction. Solid-liquid phase diagrams show the phase relationships in mixtures of two or more components and are very important in understanding the behavior of mixtures in metallurgy, material science and geology. 5.9 Liquid-solid phase diagrams Key points 1. At the eutectic composition the liquid phase solidifies without change of composition 2. The phase equilibria of binary systems in which the components react may also be summarized by a phase diagram 3. In some cases, a solid compound does not survive melting A phase diagram combines plots of pressure versus temperature for the liquid-gas, solid-liquid, and solid-gas phase-transition equilibria of a substance. These diagrams indicate the physical states that exist under specific conditions of pressure and temperature, and also provide the pressure dependence of the phase-transition temperatures ... 8 Mar 2021 — A phase diagram for two immiscible solids and the liquid phase (which is miscible in all proportions) is shown in Figure 8.9.1.
L (liquid) α (FCC solid solution) L + α l i q u i d u s s o l i d u s Cu-Ni phase diagram Phase Diagrams: # and types of phases • Rule 1: If we know T and Co, then we know:--the # and types of phases present. • Examples: A(1100°C, 60): 1 phase: α B(1250°C, 35): 2 phases: L + α Adapted from Fig. 9.3(a), Callister 7e. Elemental carbon has one gas phase, one liquid phase, and two different solid phases, as shown in the phase diagram: (a) On the phase diagram, label the gas and liquid regions. (b) Graphite is the most stable phase of carbon at normal conditions. On the phase diagram, label the graphite phase. 12.1.3 Solid-Liquid Equilibrium Phase Diagrams ... Solid-liquid equilibrium data are obtained experimentally by cooling a liquid mixture of known composition and ... by C Liu — The phase diagram belongs to a binary simple system with one eutectic point, and the content of C18-OH at eutectic point is 0.4 in mass fraction ...
2.7 Liquid solid phase diagrams · Crystallization of B releases latent heat slope of cooling curve is reduced · When reaching the eutectic line cooling stops by ...

Solid Liquid Phase Diagram Of The Binary System Octadecanoic Acid And Octadecanol And The Thermal Chemical Property Of The Composition At Eutectic Point

Use The Diagram Provided To Answer Each Of He Following Questions A Which Section Represent The Liquid Phase B Which Section Represent The Solid Phase C Which Section Represent The Gas Phase


:max_bytes(150000):strip_icc()/phase_diagram_generic-56a12a1b5f9b58b7d0bca817.png)
/phasediagram-56a129b35f9b58b7d0bca3ea.jpg)








0 Response to "37 solid liquid phase diagram"
Post a Comment