36 orbital diagram for ni
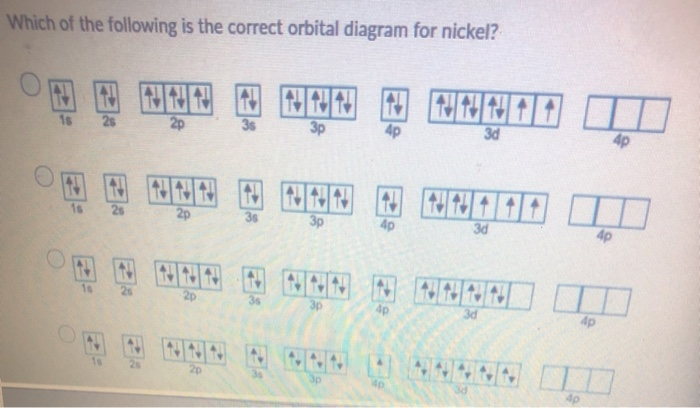
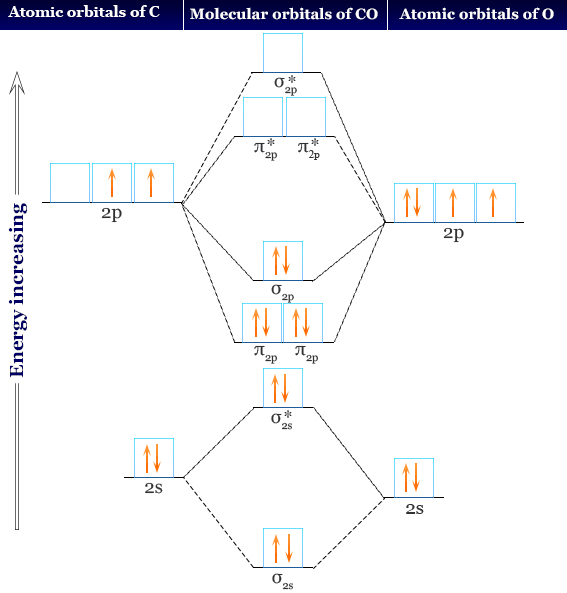
Problem: Construct the orbital diagram for Ni. FREE Expert Solution. Ni → atomic # 28 → 28 electrons. Ni will pass through 1s, 2s, 2p, 3s, 3p, 4s2, 3d. Following Aufbau principle (fill lowest energy first) and Hund's rule (half-filled first before totally filled) 82% (205 ratings) 2. Molecular orbital theory: This is the best model to explain the bonding within the CO ligand as well as in metal carbonyl complexes. There are total three molecular diagrams for carbonyl ligand which were proposed from time to time. Though, all three molecular orbital (MO) diagrams are able to explain the nature of metal-4
An orbital diagram, or orbital box diagram, is a way of representing the electron configuration of an atom. A box, line, or circle, is drawn to represent each orbital in the electron configuration. (using the Aufau Principle to order the orbitals and hence the boxes, lines or circles, as shown below) 1s. →. 2s.
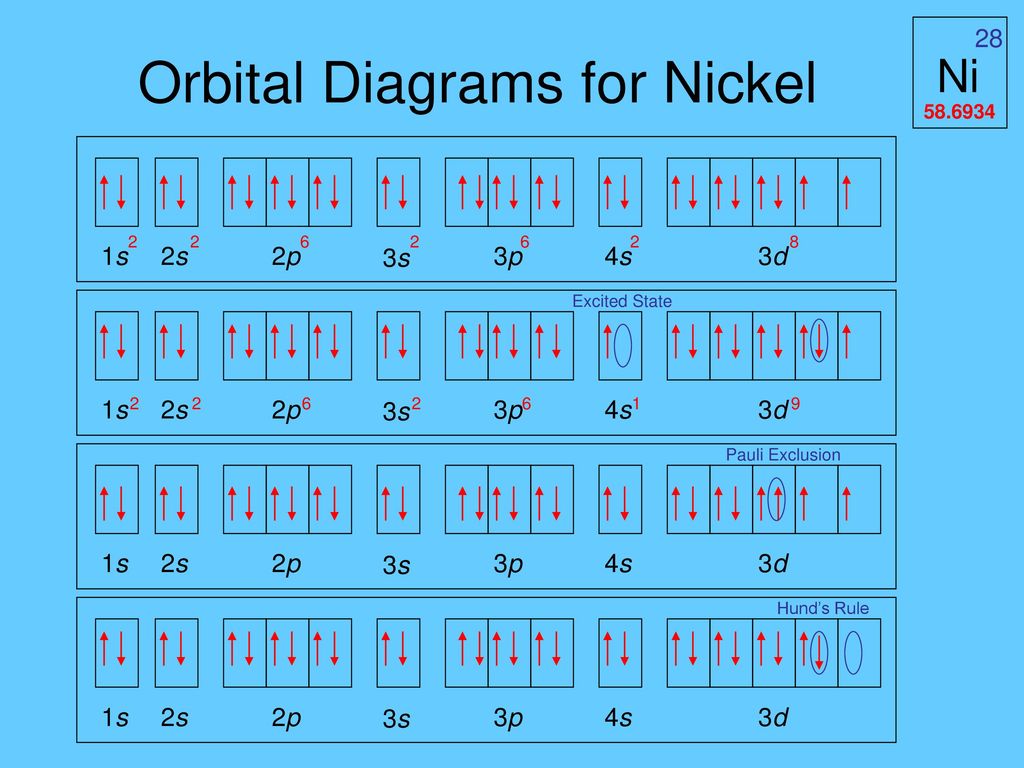
Orbital diagram for ni
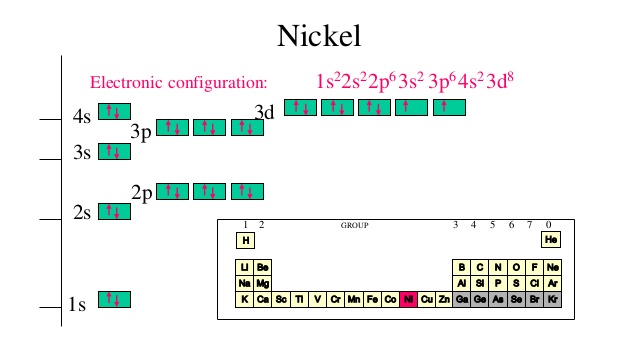
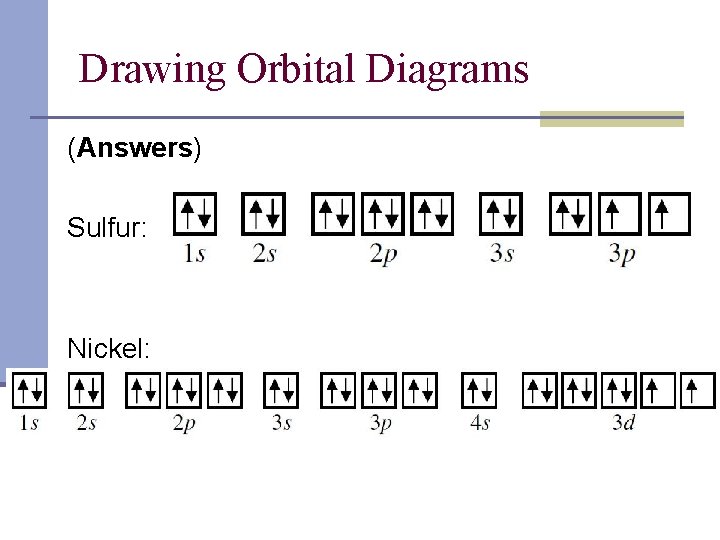
The orbital diagram for nickel is as follows: 1s2 2s2 2p6 3s2 3p64s2 3d8. In all of the cases, both up and down arrows are filled,with the exception of the 3d shell, where the last two are.What is the orbital diagram for nickel? | schematron.orgWhat is the orbital diagram for nickel. Orbital Filling Diagrams •Each box represents an orbital which can hold a max of 2 e- •Aufbau principal -each electron occupies the lowest energy orbital available; German for "build up" •Electrons are notated with an arrow -Up arrow goes first then, down arrow -Arrows represent the opposing spin of electrons 5.2 Quantum Theory & The Atom MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine
Orbital diagram for ni. Ni orbital Diagram. what is the orbital diagram for nickel answers the orbital diagram for nickel is as follows 1s2 2s2 2p6 3s2 3p64s2 3d8 in all of the cases both up and down arrows are filled with the exception of the what is the orbital diagram for nickel quora what is the orbital diagram for nickel update cancel given the rules the orbital diagram for ni is 1s2 2s2 2p6 3s2 3p6 4s2 3d8 or ... First start filling the electrons with lowest energy orbital and each orbital is singly occupied with one electron. And only two electrons with opposite spin ...1 answer · Top answer: Concepts and reason The concept used to solve this problem is based on orbital diagrams. Orbital diagrams are pictorial representation of total number ... "Ni"("CO")_4 has nickel in its 0 oxidation state, with electron configuration [Ar] 3d^8 4s^2. So, we call it a d^10 complex in the ligand field. Here is its MO diagram (it is tetrahedral): Here, the 2e and 9t_2 orbitals are what we pick out as the d-orbital splitting diagram with tetrahedral splitting energy Delta_t. The Schwarzschild metric is a spherically symmetric Lorentzian metric (here, with signature convention (−, +, +, +),) defined on (a subset of) (,)where is 3 dimensional Euclidean space, and is the two sphere. The rotation group () = acts on the or factor as rotations around the center , while leaving the first factor unchanged. The Schwarzschild metric is a solution of Einstein's field ...
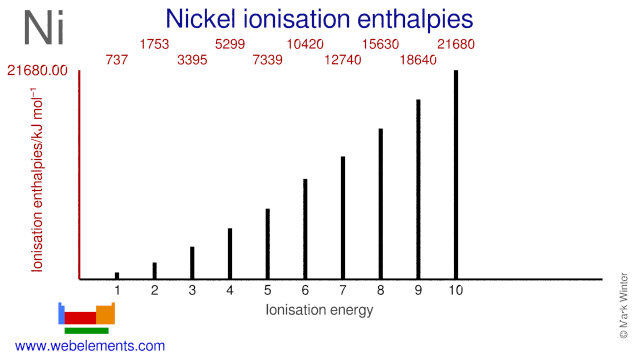
• MO diagrams can be built from group orbitals and central atom orbitals by considering orbital symmetries and energies. • The symmetry of group orbitals is determined by reducing a reducible representation of the orbitals in question. This approach is used only when the group orbitals are not obvious by inspection. Explanation: Electronic configuration of Nickel ( 28Ni) is. 1s2 2s2 2p6 3s2 3p6 4s2 3d8. After removal of two electrons from outermost shell the electron configuration of Ni2+ is. 1s2 2s2 2p6 3s2 3p6 4s0 3d8. Answer link. The fourth orbital in this subshell lies along the X and Y axes and is called the 3d x 2-y 2 orbital. Most of the space occupied by the fifth orbital lies along the Z axis and this orbital is called the 3d z 2 orbital. The number of orbitals in a shell is the square of the principal quantum number: 1 2 = 1, 2 2 = 4, 3 2 = 9. The situation with [ N i ( C N) X 4] X 2 − is that it has square planar geometry, so the two orbitals that are e g in an octahedral complex are separated in energy. This diagram from Chemistry LibreTexts shows it nicely: Because of the separation in energy, the d x y fills completely (two electrons) before any electrons fill the x 2 − y 2 ...
Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics. Jul 14, 2016 · 4 answersNickel is atomic number 28; therefore, it has 28 electrons in its orbitals. The filling rules are as follows: · Given the rules, the orbital diagram for Ni is:.What is the electron configuration of nickel?8 answersAug 10, 2015What is the electronic configuration of Ni+2?3 answersSep 1, 2017What is an abbreviated orbital diagram?3 answersApr 27, 2020More results from www.quora.com What is the orbital diagram for nickel? Atomic Orbital Diagrams: Atomic orbital diagrams are also known as electron-in-a-box diagrams. These are simplified diagrams of how electrons are arranged ... Answer and Explanation: 1. To help determine the electron configuration of nickel (Ni), we will visualize how the electrons are distributed in an Aufbau diagram below. Nickel has an atomic number ...
Jan 26, 2021 — Valence electrons are the electrons which are located in the outer shell or orbit. There are 28 electrons in the nickel in the 4 orbits and the ...

Theoretical Study Of One Electron Oxidized Salen Complexes Of Group 7 Mn Iii Tc Iii And Re Iii And Group 10 Metals Ni Ii Pd Ii A
Answer to Write orbital diagrams for each of these ions. V5+,Cr3+,Ni2+,Fe3+ Determine if the ion is diamagnetic or paramagnetic. V 86%(14). Write orbital diagrams for each of these ions. A. V^5+ B. Cr^3+ C. Ni^2+ D. Fe^3+ E. Determine if the following ions are diamagnetic or paramagnetic. V5+ orbital diagram keyword after analyzing the system ...
This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. It explains how to write the orbital diagram n...
Orbital diagram of Nickel (Ni) 29: Orbital diagram of Copper (Cu) 30: Orbital diagram of Zinc (Zn) 31: Orbital diagram of Gallium (Ga) 32: Orbital diagram of Germanium (Ge) 33: Orbital diagram of Arsenic (As) 34: Orbital diagram of Selenium (Se) 35: Orbital diagram of Bromine (Br) 36: Orbital diagram of Krypton (Kr) 37: Orbital diagram of ...
For the orbital diagram, we simply need to draw each orbital and fill them up with the correct number of electrons. Take note that we need to ...Aug 27, 20181 answer · Top answer: We’re being asked to construct the orbital diagram for Ni. For that, we first need to determine the electron configuration of Ni.Recall that for a ...
In this case, the d z 2 orbital drops even lower in energy, and the molecule has the following orbital splitting diagram. As a result of these distortions, there is a net lowering of energy (an increase in the ligand field stabilization energy) for complexes in which the metal has a d 7 , d 8 , or d 9 configurations, and thus electrons would ...
Nickel (Ni) has an atomic mass of 28. Find out about its chemical and ... Electron Configuration, [Ar] 3d8 4s2. 1s2 2s2 2p6 3s2 3p6 3d8 4s2. Orbital Diagram.
The orbital diagram for nickel is as follows: 1s2 2s2 2p6 3s2 3p6 4s2 3d8. In all of the cases, both up and down arrows are filled, with the exception of the 3d shell, where the last two are up ...
Orbital diagrams are pictorial descriptions of the electrons in an atom. Three rules are useful in forming orbital diagrams. According to the Auf Bau Principle, each electron occupies the lowest energy orbital. You jump up a little bit in energy and we get the 2s orbital that make it the 2p sublevel.
Answer (1 of 4): Nickel is atomic number 28; therefore, it has 28 electrons in its orbitals. The filling rules are as follows: 1. Aufbau Principle: Lowest energy levels fill first. 2. Pauli Exclusion Principle: Only 2 electrons per orbital, they must have opposite spin. 3. Hund's Rule: Given sev...

Tuliskan Konfigurasi Elektron Dan Diagram Orbital Berdasarkan Teori Mekanika Kuantum Untuk Unsur Brainly Co Id
Fluorine (F) Electron Configuration with Full Orbital Diagram. Fluorine electron configuration is 1s 2 2s 2 2p 5. The symbol for fluorine is F. The period of fluorine is 2 and it is a p-block element. The electron configuration of fluorine (F) and the orbital diagram is the main topic of this article.
Interactive 3D chemistry animations of reaction mechanisms and 3D models of chemical structures for students studying University courses and advanced school chemistry ...
Electron configuration for Ni is 1s22s22p64s23d8 . Ni2+ has two electrons less than Ni ( that is why Ni2+ is positively charged). So when writing electron ...2 answers · 1s22s22p63s23p64s23d6 in the ground state More correctly the electrons lost are the 4s2leaving ...
Transcribed Image Textfrom this Question. Construct the orbital diagram for nickel 1 11 1 4p 1 11 14 1 3d 1 4s Answer Bank 1 1 1 3p 1 1 1L 3s 1 1 1L 2p 1 2s 1s Energy Construct the orbital diagram of the F ion Зр Answer Bank 3s 1 2p 2.s 1s Energy.

Ni Nickel Element Information Facts Properties Trends Uses And Comparison Periodic Table Of The Elements Schoolmykids
Nickel electron configuration. ← Electronic configurations of elements. Ni (Nickel) is an element with position number 28 in the periodic table. Located in the ...

Write The Abbreviated Orbital Diagrams For The Following Elements And State Whether They Are Paramagnetic Or Diamagnetic A Ni 2 B Ca 2 Study Com
Answer to Construct the orbital diagram for Ni. Start by adding the appropriate subshells. For example, carbon is in the 2p block.1. Describe the two differences between a 2p x orbital and a 3p y orbital. The 2px orbital lies on the x-axis. The 3py orbital lies on the y-axis and is larger than the 2px orbital. 2.
Hund©s Rule & Orbital Filling Diagram Complete the orbital diagram for each element. 2) calcium 1s 2s 4s 3s 3d 2p 4p 3p 1) sodium 1s 2s 4s 3s 3d 2p 4p 3p 3) nickel 1s 2s 4s 3s 3d 2p 4p 3p 4) silicon 1s 2s 4s 3s 3d 2p 4p 3p 5) iron 6) copper 1s 2s 4s 3s 3d 2p 4p 3p 2s2 2p6 3s2 3p6 4s2 1s 2s 4s 3s 2p 4p 3p 3d. Name : Printable Worksheets @
The orbital diagram for nickel is as follows. What is the orbital diagram for nickel. In all of the cases both up and down arrows are filled with the exception of the 3d shell where the last two are up. Were being asked to construct the orbital diagram for nifor that we first need to determine the electron configuration of ni.
Answer (1 of 7): Since there are 2 electrons which are left unpaired, the molecule is paramagnetic in nature.

Figure 4 From A Study Of Nickel Monoxide Nio Nickel Dioxide Onio And Ni O2 Complex By Anion Photoelectron Spectroscopy Semantic Scholar
Orbital diagrams are a visual way to show where the electrons are located within an atom. Orbital diagrams must follow 3 rules: The Aufbau principle, the Pau...
La Niña (/ l ə ˈ n i n. j ə /; Spanish: ) is an oceanic and atmospheric phenomenon that is the colder counterpart of El Niño, as part of the broader El Niño–Southern Oscillation (ENSO) climate pattern.The name La Niña originates from Spanish for "the girl", by analogy to El Niño, meaning "the boy".In the past, it was also called an anti-El Niño and El Viejo, meaning "the old man".
MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine
Orbital Filling Diagrams •Each box represents an orbital which can hold a max of 2 e- •Aufbau principal -each electron occupies the lowest energy orbital available; German for "build up" •Electrons are notated with an arrow -Up arrow goes first then, down arrow -Arrows represent the opposing spin of electrons 5.2 Quantum Theory & The Atom
The orbital diagram for nickel is as follows: 1s2 2s2 2p6 3s2 3p64s2 3d8. In all of the cases, both up and down arrows are filled,with the exception of the 3d shell, where the last two are.What is the orbital diagram for nickel? | schematron.orgWhat is the orbital diagram for nickel.

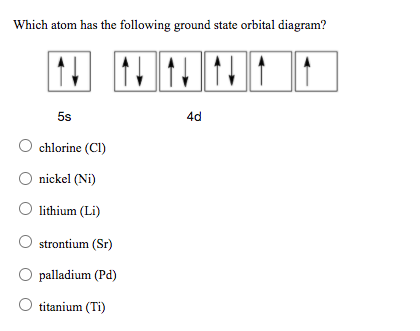

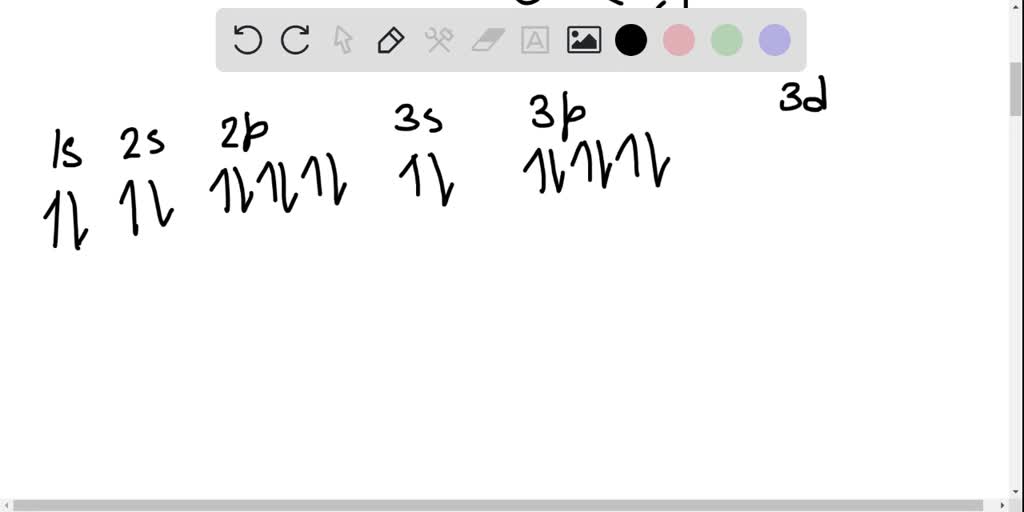



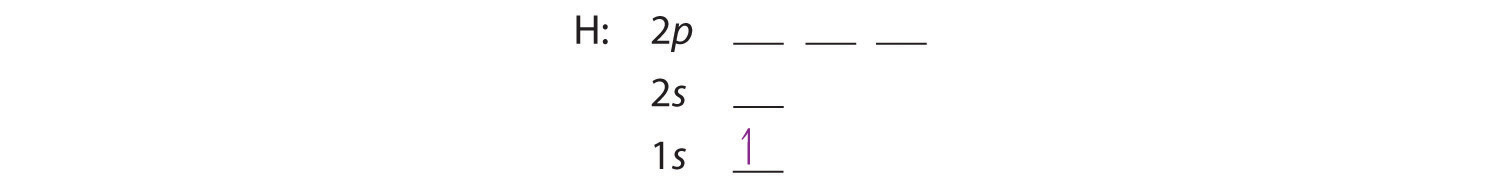












0 Response to "36 orbital diagram for ni"
Post a Comment