36 lewis dot diagram for aluminum
From Wikipedia, the free encyclopedia Jump to navigationJump to search This article is about the cat species that is commonly kept as a pet. For the cat family, see Felidae. For other uses, see Cat (disambiguation) and Cats (disambiguation). For technical reasons, "Cat #1" redirects here. For the album, see Cat 1 (album). Domestic cat[1] Cat poster 1.jpg Various types of domestic cat Conservation status Domesticated Scientific classification e Kingdom: Animalia Phylum: Chordata Class: Mammalia O... By going through the periodic table, we see that the Lewis electron dot diagrams of atoms will never have more than eight dots around the atomic symbol. ... The valence electron configuration for aluminum is 3s23p1. So it would have three dots around the symbol for aluminum, two of them paired ...
Fill in the lewis dot symbols for. Sodium reacts explosively with chlorine gas to form sodium chloride. Lewis Diagrams Ionic And Covalent Bonds Worksheet Thursday December 6 2018 Covalent Bonding Worksheet Covalent Bonding Science Worksheets Zinc or aluminum if h 2 gas tank is not available 4 6m hcl if h 2 gas tank is […]

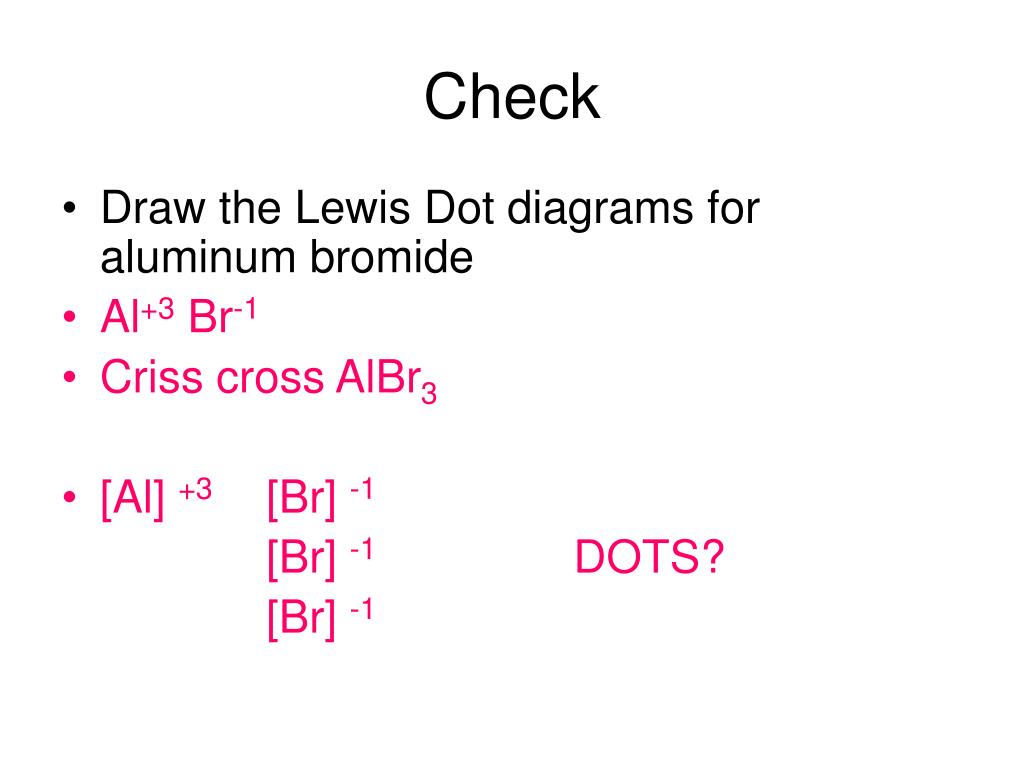
Lewis dot diagram for aluminum
Aluminum Lewis Dot Structure What is Lewis' symbol for Al? | Then I draw the Lewis point structure for aluminum (Al). Note: Aluminum belongs to group 13 (also called group III or 3A). Since it is part of group 3, it has 3 valence electrons. When drawing the Lewis structure for aluminum, place three points or valence electrons around the symbol (Al). And what is the Lewis structure of Al ... April 11, 2021 - After that I draw the Lewis dot structure for Aluminum (Al). Note: Aluminum is in Group 13 (sometimes called Group III or 3A). Since it is in Group 3 it will have 3 valence electrons. When you draw the Lewis structure for Aluminum you'll put three 'dots' or valance electrons around the element ... April 14, 2019 - Aluminum lewis dot structure. Which of these is the correct lewis dot diagram for aluminum. Aluminum Chloride Lewis Dot Str...
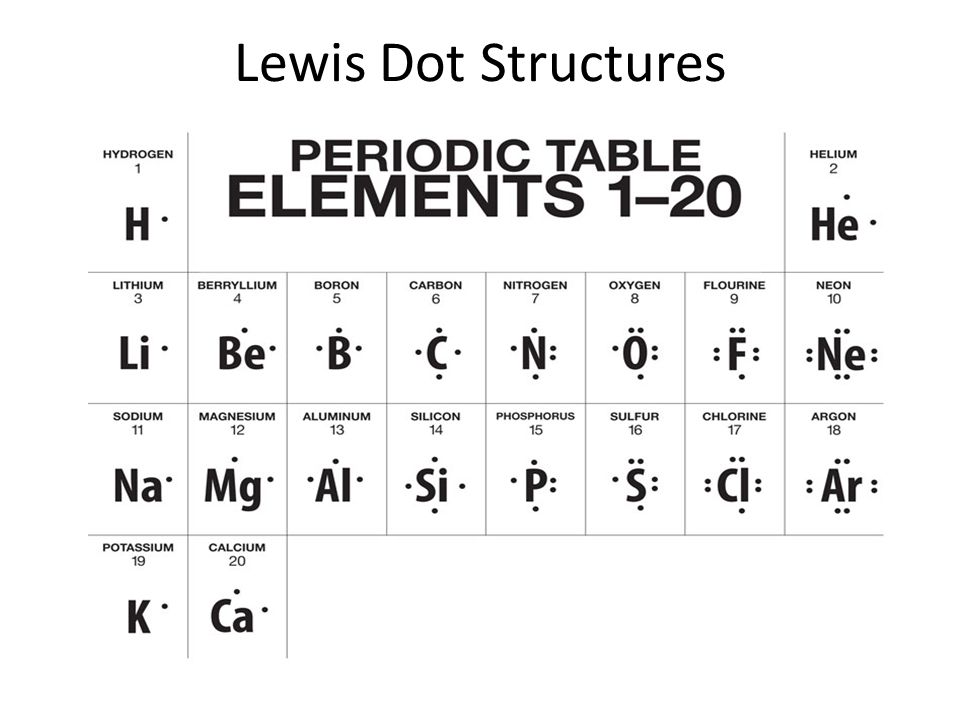
Lewis dot diagram for aluminum. The Lewis dot structure for an atom of sodium is The number of electrons sodium has per shell is 2-8-1, therefore it has one valence electron, and the diagram needs one dot. Since bonding involves the valance shell electrons only, it is only necessary to illustrate those outer electrons. 150 m 2 g −1) , . E +100 PTS. 1. draw the Lewis dot structure for Lead(IV) Fluoride and predict its electronic and molecular structure. Begin with the valence electrons for each of the elements in the compound, getting the total valence electrons for the compound, writing the number of electron groups, bonding groups and lone pairs of electrons on the central atom. Example \(\PageIndex{1}\): What is the Lewis electron dot diagram for each element? aluminum; selenium; Solution. The valence electron configuration for aluminum is 3s 2 3p 1.So it would have three dots around the symbol for aluminum, two of them paired to represent the 3s electrons: \[\dot{Al:} \nonumber\nonumber \] A Lewis Structure is a very simplified representation of the valence shell electrons in a molecule. It is used to show how the electrons are arranged around individual atoms in a molecule. Electrons are shown as "dots" or for bonding electrons as a line between the two atoms.What is the Lewis dot structure for TE?
A step-by-step explanation of how to draw the Lewis dot structure for Al (Aluminum). I show you where Aluminum is on the periodic table and how to determine ... The Lewis dot structure for aluminum includes the symbol, "Al," and a total of three dots around the symbol. a. True. b. False. Lewis Structure: Lewis Dot ... A step-by-step explanation of how to draw the Al2O3 Lewis Dot Structure.For Al2O3 we have an ionic compound and we need to take that into ... Figure 2. Cations are formed when atoms lose electrons, represented by fewer Lewis dots, whereas anions are formed by atoms gaining electrons. The total number of electrons does not change. ... The valence electron configuration for aluminum is 3s23p1. So it would have three dots around the ...
Lewis Dot Structure for Francium For example, an element in group 1A will have one valence electron, and therefore, one dot. An element in group 2A will have two valence electrons, and (you ... CHAPTER 7. A (n) _______ bond is a chemical bond that results from sharing a pair of electrons between two atoms. A (n) ______ results from a transfer of one or more electrons from one atom or molecule to another. Nice work! You just studied 91 terms! Now up your study game with Learn mode. Lg Lsc26905Tt Ice Maker Diagram. Download Lg Lsc26905Tt Ice Maker Diagram PNG . Wiring diagram 31 lg ice maker parts diagram. Lg lsc26905tt ice maker diagram…. July 12, 2021 Add Comment. Edit. Example \(\PageIndex{1}\): Lewis Dot Diagrams. What is the Lewis electron dot diagram for each element? aluminum; selenium; Solution. The valence electron configuration for aluminum is 3s 2 3p 1.So it would have three dots around the symbol for aluminum, two of them paired to represent the 3s electrons: \[\dot{Al:} \nonumber\]
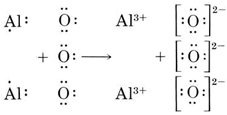
No Comments on Lewis Structure of Al2O3, Aluminum Oxide Aluminium oxide is a solid ionic compound, made from atoms of one metal (Aluminum) that have lost three electrons each to become +3 cations, and atoms of a non-metal (oxygen) which have gained two electrons each to become -2 anions.
By going through the periodic table, we see that the Lewis electron dot diagrams of atoms will never have more than eight dots around the atomic symbol. ... The valence electron configuration for aluminum is 3s23p1. So it would have three dots around the symbol for aluminum, two of them paired ...
ShowMe is an open learning community featuring interactive lessons on a variety of topics.
How many electrons are represented in the Lewis dot structure for aluminum (Al)? 3. Which of the following is MOST likely an exothermic chemical change? burning paper. Which can be concluded from the phase change graph? From time G to H, heat energy was absorbed.
Find an answer to your question what type of bond does carbon and sulphur form when they react. explain
An atom's Lewis dot structure has four dots. Which of the following elements could it be, and why? O Aluminum, because it is a metal with four electrons in period 3. O Beryllium, because it is in period 2 and has four total electrons. O Carbon, because it is in group 14 and has four … Continue reading "An atom's Lewis dot structure has four dots.
February 1, 2020 - To draw the Lewis electron dot diagram we picture in our minds the symbol for Mg in a box with all of its core electrons (i.e., 1s22s22p6). Then we place the valence electrons around the sides of the box with each side representing an orbital in the outermost energy level. ... Aluminum oxide is ...

Chemistry Chemical Bonding 18 Of 35 Lewis Structures For Ionic Compounds This Lecture By Michel Van Bie Chemistry Education Chemistry Worksheets Chemistry
Which statement describes a chemical property of aluminum? (1) Aluminum has a density of 2.698 g/em at STP. (2) Aluminum reacts with sulfuric acid. (3) Aluminum conducts an electric current. (4) Aluminum is malleable.

Today Is Thursday October 16 Th 2014 Pre Class When We Were Naming Things Like Co 2 And Hno 3 And Srf 2 What Were All The Atoms In Those Compounds Ppt Download
Sign in|Recent Site Activity|Report Abuse|Print Page|Powered By Google Sites
Estructuras de Lewis y Regla del Octeto ejemplos y excepciones a la reglaocteto incompleto octeto expandido número impar de electrones. A step-by-step explanation of how to draw the AlCl3 Lewis Dot Structure Aluminum chlorideFor the AlCl3 structure use the periodic table to find the total.
Atomic Structure Links · Valence Electrons and Lewis Electron Dots of Atoms and Ions
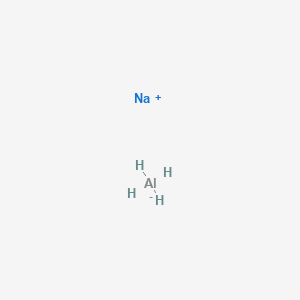
Once we know how many valence electrons there are in Aluminum (Al) we can figure out the Al3+ Lewis dot structure. Since the Aluminum ion is ...
By going through the periodic table, we see that the Lewis electron dot diagrams of atoms will never have more than eight dots around the atomic symbol. ... a) The valence electron configuration for aluminum is 3s23p1. So it would have three dots around the symbol for aluminum, two of them ...
Explain How To Draw A Dot And Cross Diagram Which Shows The Ionic Bonding In Aluminum Oxide A L 2 O 3 Study Com
An atom's Lewis dot structure has three dots. Which of the following elements could it be, and why? (4 points) Group of answer choices Aluminum, becau … se it is in group 13 and has three valence electrons. Lithium, because it is a group 1 element with three total electrons. Magnesium, because it is in period 3 and has three valence electrons.
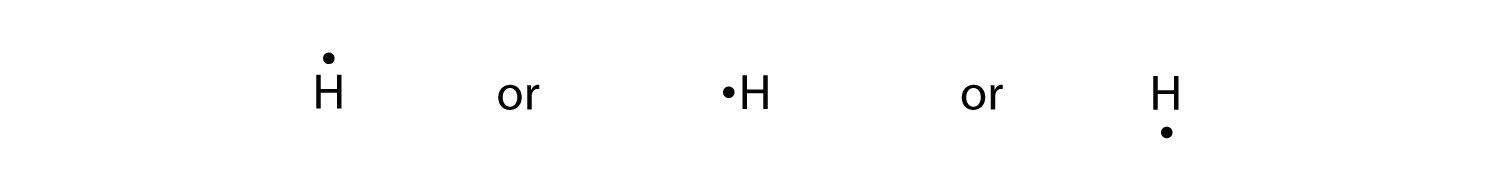
Hint: We can say that electron dot structure is nothing but a representation of Lewis dot diagram of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots indicates the number of outermost electrons present in the atom.
Step 3: Lewis Structure is also known as an electron-dot structure since we use dot notations to represent the valence electrons surrounding the atoms. Let us have a look at the schematic sketch of HF after we have placed the dot electrons: Step 4: Now, we will check the octet rule.
A step-by-step explanation of how to draw the CS2 Lewis Dot Structure Carbon disulfideFor the CS2 structure use the periodic table to find the total numbe. Aluminum 13 3s2 p1 Al 3. I am looking at drawing the lewis structure for the S2- ion.
Lewis Dot Diagram Aluminum Oxide Electron For Michaelhannan Co Exercises explain why the first two dots in a lewis electron dot diagram are drawn on the same side of the atomic symbol. Lewis dot diagram for neon. This function creates a molecule that represents the input molecule as a lewis dot structure using 90 angles and appropriately place ...
Read also which and which equation demonstrates the additive identity property So 0 is the additive identity here. For example 120 0 120 illustrates identity property of addition where 0 is the additive identity. Here are some examples involving whole numbers. On Distributive Property Take for instanceThere are uncountable number of examples.
June 22, 2016 - Something went wrong. Wait a moment and try again
BCl3 Lewis Structure. Let us apply the lewis dot rules and try to draw the structure of boron trichloride. First of all, we need to calculate the total valence electrons of this molecule, B = 3. C l= 7. 3Cl = 7*3=21. So, total= 21+3= 24. Now, boron is less electronegative, which makes it the central atom.

What Is The Electron Dot Structure Of Aluminium Oxide And Calcium Nitrate Science Metals And Non Metals 13062881 Meritnation Com
Solve the Lewis structure and check your answer with a video explanationLewis Structures Worksheet PracticeThe more Le. Sodium to chlorine can be depicted by a Lewis dot diagram. Element atomic atomic mass protons neutrons electrons lewis dot carbon 6 12 6 6 6 l hydrogen 1 1 1 0 1 h lithium 3 7 3 4 3 li.
Beginne mit Electron Dot Diagrams. Lerne Vokabeln, Begriffe und weitere Inhalte mit Karteikarten, Spielen und anderen Lerntools.
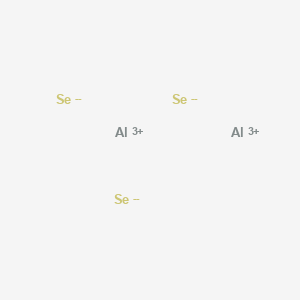
Answer to Write the Lewis structure for each ion.(a) Al3+(b) Mg2+(c) Se2−(d) N3−.
An electron dot structure is depicted using the element symbol and then starting at the top of the element symbol, dots are added in a clockwise fashion until the number of valence electrons has been reached. The maximum number of electrons is limited to eight. ... Aluminum is in group IIIA ...
March 24, 2021 - By going through the periodic table, we see that the Lewis electron dot diagrams of atoms will never have more than eight dots around the atomic symbol. ... . So it would have three dots around the symbol for aluminum, two of them paired to represent the 3s electrons:
Draw The Lewis Structure Of Mgbr2 Magnesium Bromide Youtube . Magnesium has an electronic configuration of 282. Magnesium bromide electron configuration. 47 1959 x 2 of 4 Describe the electron transfers that occur in the formation of magnesium bromide from elemental magnesium and elemental bromine. Hence it has a charge of 2 Mg2.

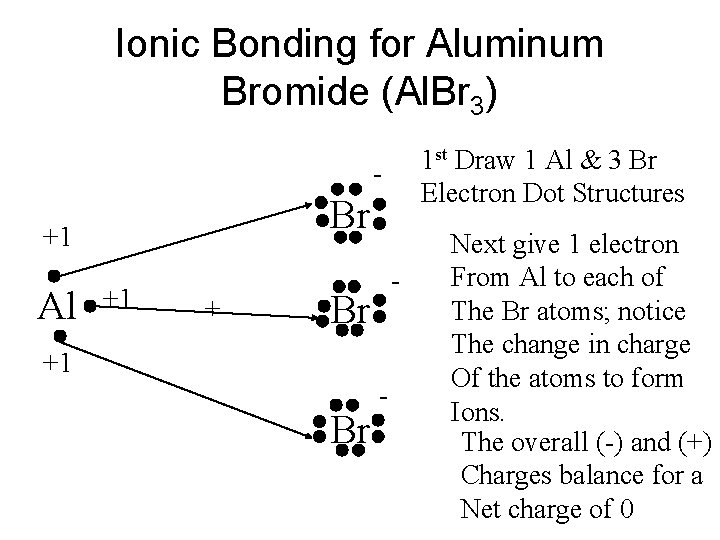
Draw The Lewis Structure For Aluminum Sulfide Ionic Compound And What Chemical Formula The Lewis Theory Predicts Study Com
Aluminum nitride powder -200 mesh 998 trace metals basis. In this lesson we will discuss how to determine the formula for aluminum nitrate what it is used for and its solubility. 2 Learn The Criss Cross Method Ppt Download . How To Draw The Lewis Dot Structure For Al No3 3 Aluminum Nitrate Youtube . How To Write The Formula For Aluminum Nitride ...
The ionic formula for calcium oxide is simply cao. Based on the dot diagram for the atoms in exercise 4, identify what you expect the charges on calcium ions and oxide ions to be when they form compounds. Buy lewis dot structure for ch2chcl lewis dot structure for note that cao is also called calcium oxide.
5. In a Lewis structure, each pair of electrons shared between nuclei (l. bonding pairs) may be represented by a line rather than dots. Any unshared electrons (i.e., nonbonding pairs) are usually represented as dots. Use the electron dot structure of the compound below to answer the following questions.
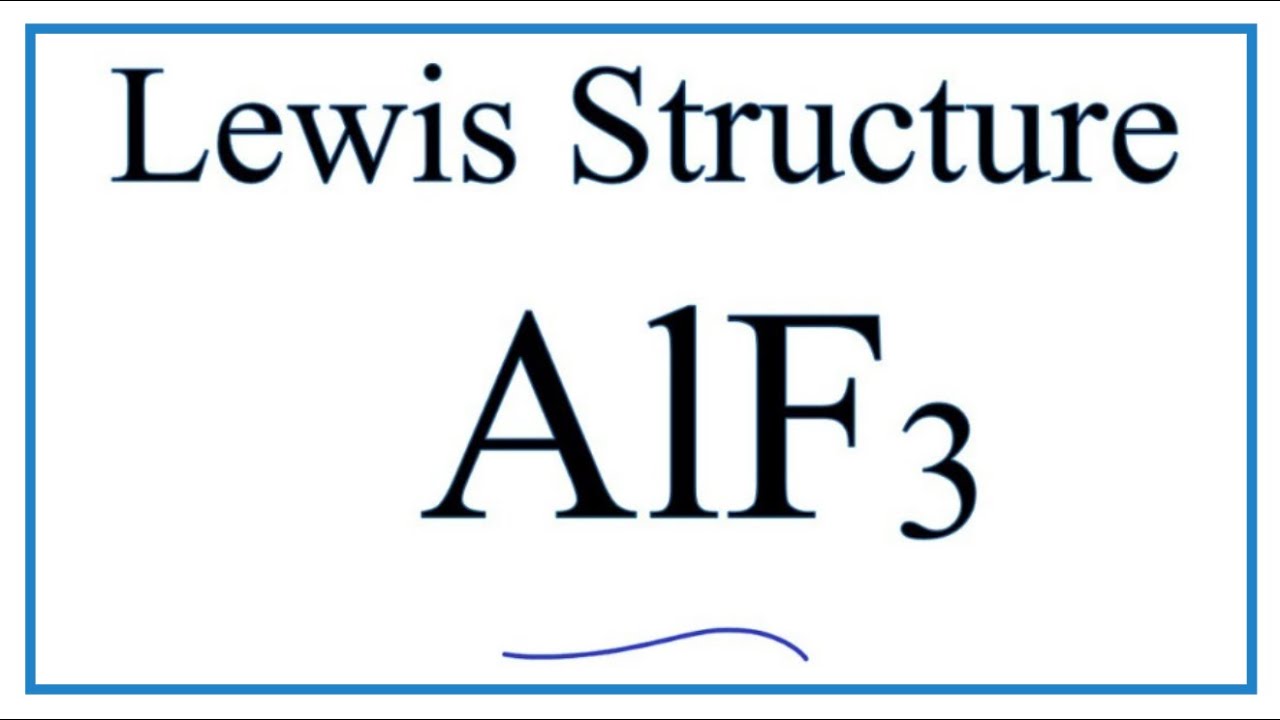
Estructura de lewis de AlF3 Recibe ahora mismo las respuestas que necesitas. Por su gran variedad isomérica no es posible. A step-by-step explanation of how to draw the AlCl3 Lewis Dot Structure Aluminum chlorideFor the AlCl3 structure use the periodic table to find the total. Las principales propiedades del fluoruro alumínico AlF3 son.
October 26, 2019 - SO2 Lewis structure (sulfur dioxide electron dot structure) is that type of diagram where we show the total 18 valence electrons of SO2 as dots , or dots and dashes(-).In Lewis structure,it is common that a bonding pair of two electrons can be shown by dash(-) or dots(●●) but a lone pair ...
April 14, 2019 - Aluminum lewis dot structure. Which of these is the correct lewis dot diagram for aluminum. Aluminum Chloride Lewis Dot Str...
April 11, 2021 - After that I draw the Lewis dot structure for Aluminum (Al). Note: Aluminum is in Group 13 (sometimes called Group III or 3A). Since it is in Group 3 it will have 3 valence electrons. When you draw the Lewis structure for Aluminum you'll put three 'dots' or valance electrons around the element ...
Aluminum Lewis Dot Structure What is Lewis' symbol for Al? | Then I draw the Lewis point structure for aluminum (Al). Note: Aluminum belongs to group 13 (also called group III or 3A). Since it is part of group 3, it has 3 valence electrons. When drawing the Lewis structure for aluminum, place three points or valence electrons around the symbol (Al). And what is the Lewis structure of Al ...
















0 Response to "36 lewis dot diagram for aluminum"
Post a Comment