36 f2 molecular orbital diagram
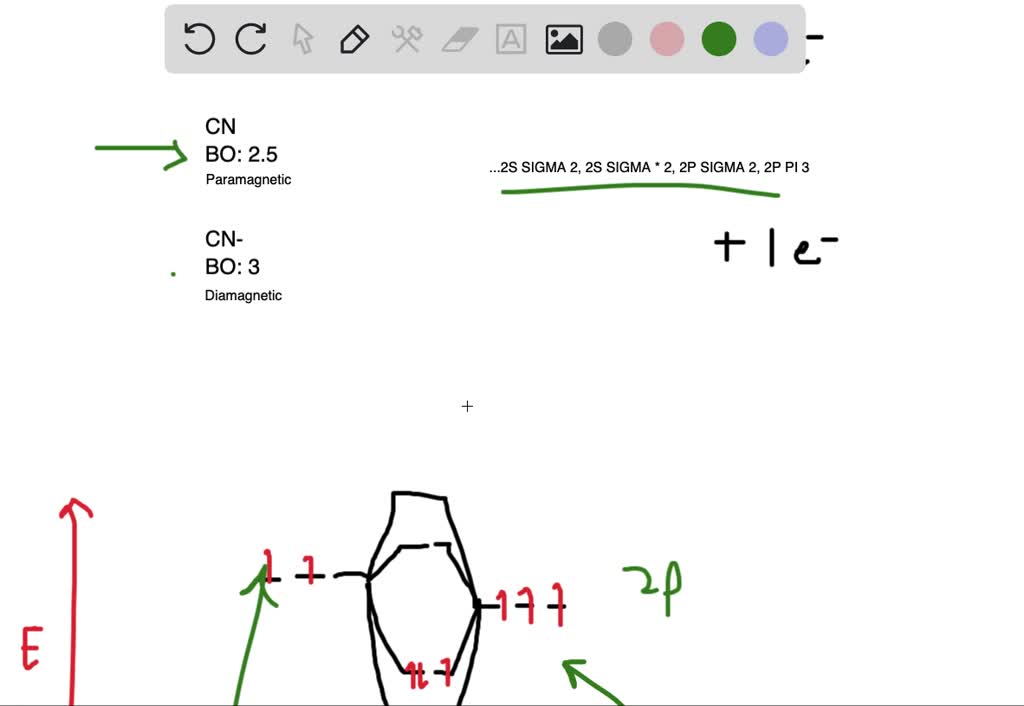
About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators ... Answer (1 of 4): The atomic number of fluorine is 9, so a (neutral) F2 molecule has a total of 18 electron, or 14 valence electrons (excluding the four 1s electrons). The (F2)- ion has one more valence electron, or 15. The orbital diagram for a diatomic molecule is To find the bond order, add th...
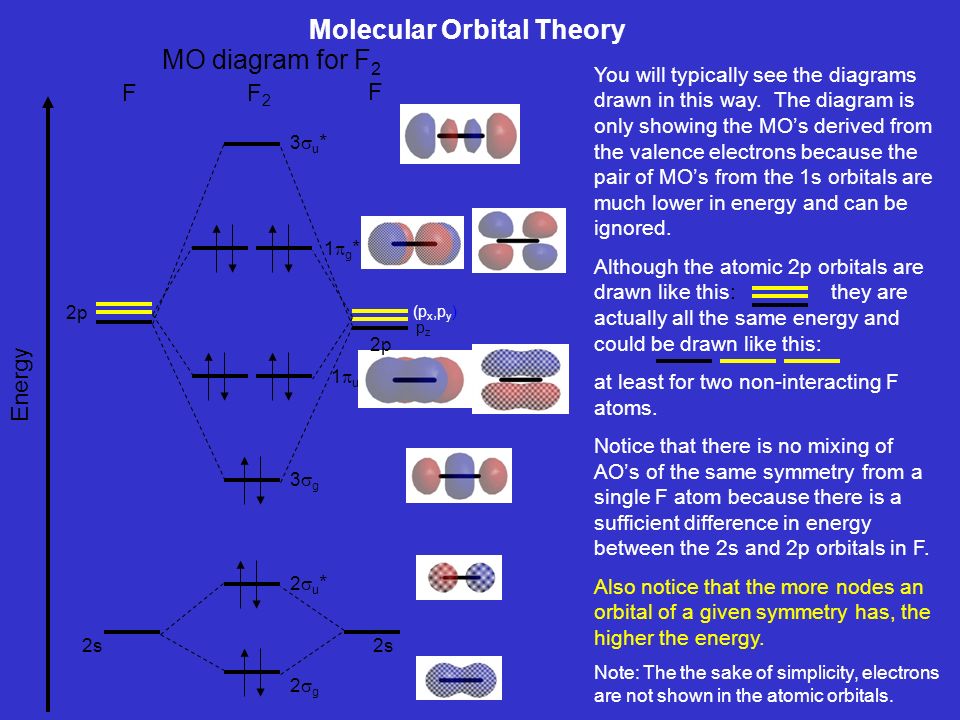
Summary MO Theory • LCAO-MO Theory is a simple method for predicting the approximate electronic structure of molecules. • Atomic orbitals must have the proper symmetry and energy to interact and form molecular orbitals. • Photoelectron spectroscopy provides useful information on the energies of atomic orbitals. • Next we'll see that symmetry will help us treat larger molecules in
F2 molecular orbital diagram
Molecular Orbital Energy Diagram For F2 Energy Etfs How To Draw Molecular Orbital Diagram 142452 Paramagnetism And What Is The Molecular Orbital Diagram Of O2 And F2 Quora Mo Diagram 2 F2 Youtube Molecular Orbital Theory C2 Molecule Doubly Or Quadruply Bonded Mapping Ignorance Quiz 3 Molecular Orbitals Che 331 Molecular Science Ii Studocu ... Before we can draw a molecular orbital diagram for B₂, we must find the in-phase and out-of-phase overlap combinations for boron's atomic orbitals. Then we rank them in order of increasing energy. We can ignore the 1s orbitals, because they do not contain the valence electrons. Each boron atom has one 2s and three 2p valence orbitals. The 2s orbitals will overlap to form 2sσ and 2sσ ... Molecular ion (He2)+ has a bond order of 0.5 , while (H2)+ has a bond order 0.5. He2+ is the more stable of the pair because it has two electrons that it can release to form the ion. Is F2 or F2+ more stable? Between F2, F2+, and F2_ which do you think would have the highest bond order, strongest bond, longest bond length.
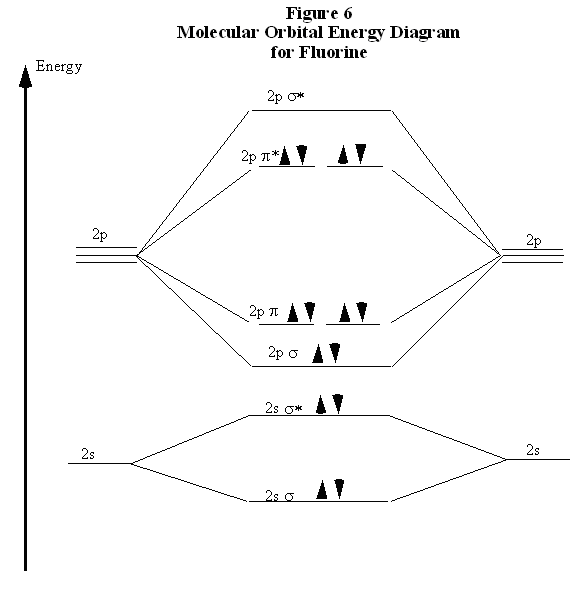
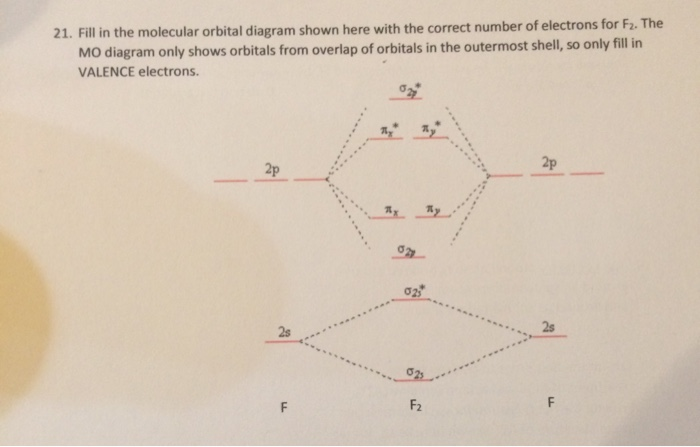
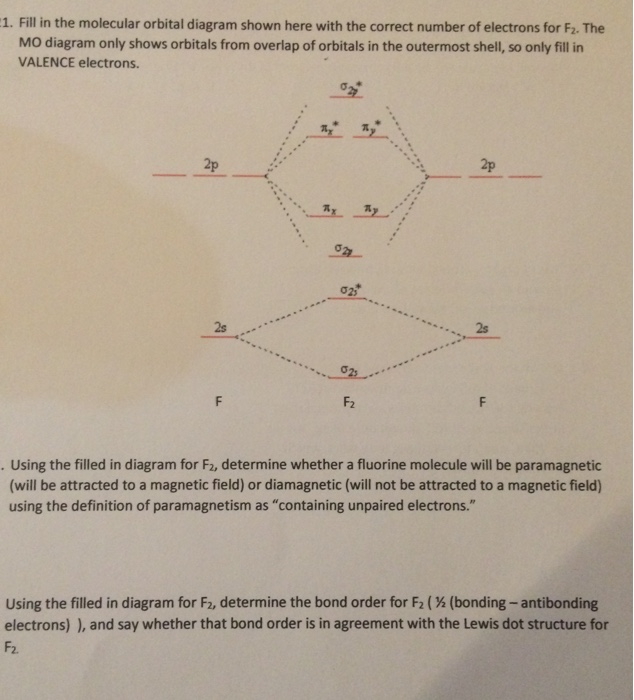
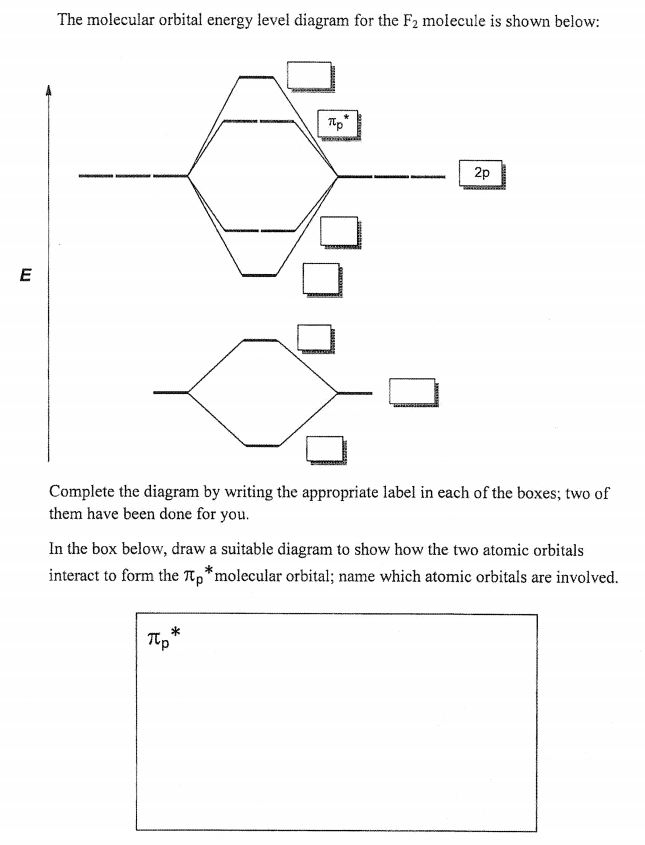
F2 molecular orbital diagram. Click here👆to get an answer to your question ️ 37. Draw molecular orbital diagram for F2 molecule. Also, give its electronic configuration, bond order and magnetic property. 138. Solve the following: Transcribed image text: Jestion 11 (20 pts.) . Complete the molecular orbital diagram for F2. Do not include the inner shell electrons. (5 pts.) b. Count the number of valence electrons in F2, (2 pts.) Molecular Orbital Diagram - Cl2, Br2, I2 3s & 3p and higher atomic orbitals are not so widely separated in energy and allow significant mixing (hybridization) to occur. This mixing causes the inversion of the σσand πmolecular orbitals' energy. σσσ ππ σ* π* 3,4,5 p 3, 4,5 s σ* σ 3,4,5 s 3,4,5 p Interhalogens Br Br F F Br F F F F. The case of F2 is a simple one because of the symmetry and diatomicity of the molecule. In more complex molecules (polyatomic and asymmetric), the extent of mixing and thus the contribution of individual atomic orbitals to form a particular molecular orbital depends on the relative energy alignment of the atomic orbitals. F2 Polarity
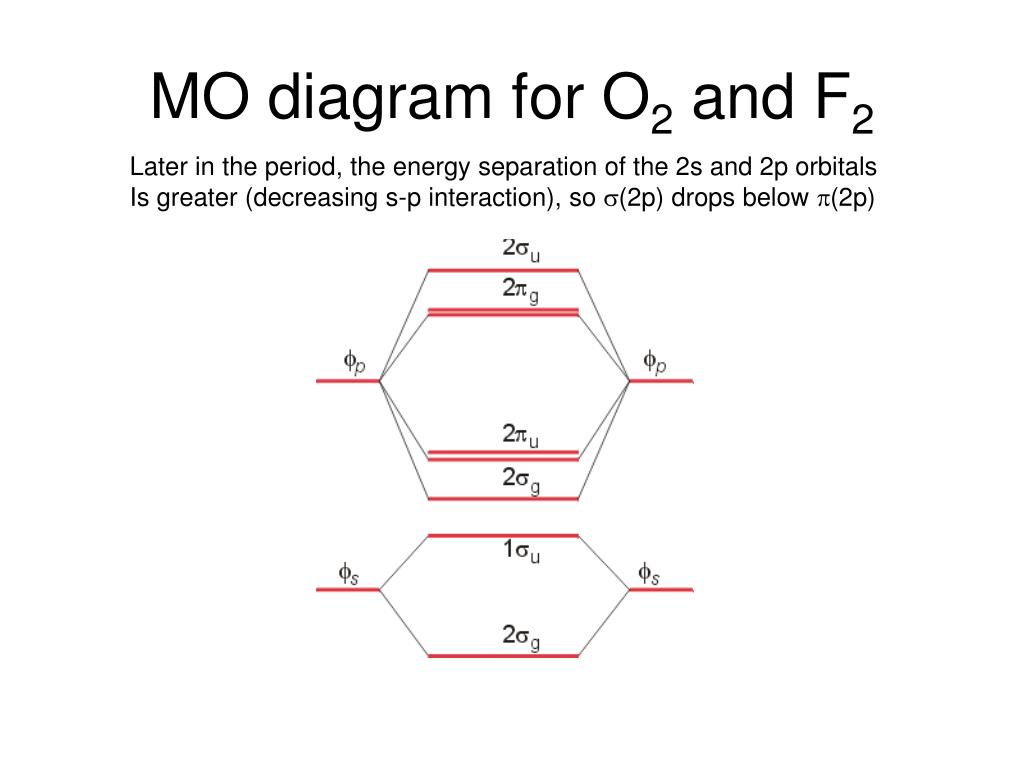
molecular orbitals in the diagram suggest a double bond. c. The 2s, 2s *, 2p, and 2p * orbitals exhibit C v symmetry, with the NF bond axis the infinite-fold rotation axis. The 2p and 2p * orbitals exhibit Cs symmetry. The latter do not possess C2 rotation axes coincident to the Molecular orbital diagram for f2. In o 2 and f 2 there is a crossover of the sigma and the pi ortbials. Molecular orbitals mo are constructed from atomic orbitals. The relative energies of the sigma orbitals drop below that of the pi orbitals. The size of the effect depends on the 2s 2p energy difference. It is called a sigma molecular orbital ... Sep 30, 2017 · A bare molecular orbital diagram is presented and you must drag the correct orbitals and labels onto the diagram. Molecular orbital diagram for f2. The other molecular orbital produced s h h shows a decrease in electron density between the nuclei reaching a value of zero at the midpoint between the nuclei where there is a nodal plane. Draw molecular orbital diagram for F2 molecule Also gives its electronic configuration bond order and magnetic property. Claim your FREE Seat in Vedantu Master Classes! ... -MOT uses a linear combination of atomic orbitals strategy to represent molecular orbitals resulting from bonds between atoms. These are bonding, anti-bonding and non-bonding.
Molecular orbital diagram for f2. A bare molecular orbital diagram is presented and you must drag the correct orbitals and labels onto the diagram. How to graph a mo molecular orbital diagram for f2. By drawing molecular orbital diagrams for b2 c2 n2 o2 and f2 predict which of these homonuclear diatomic molecules are magnetic. Molecular Orbitals of the Second Energy Level. The 2s orbitals on one atom combine with the 2s orbitals on another to form a 2s bonding and a 2s * antibonding molecular orbital, just like the 1s and 1s * orbitals formed from the 1s atomic orbitals. If we arbitrarily define the Z axis of the coordinate system for the O 2 molecule as the axis along which the bond forms, the 2p z orbitals on the ... Molecular orbital diagram for f2. A fundamental principle of these theories is that as atoms bond to form molecules a certain number of atomic orbitals combine to form the same number of. C would this ion exist. To further demonstrate the consistency of the lewis structures with mo. For the ion f2. Relationship between electronic configuration and Molecular behaviour. 1) Stability of molecules in terms of bonding and antibonding electrons . Number of electrons present in the bonding orbitals is represented by N b and the number of electrons present in antibonding orbitals by Na.. 1) If N b > Na,the molecule is stable because greater number of bonding orbitals are occupied than ...
Now, let us draw the molecular orbital diagram of ${N_2}$ . Now, first let us understand what magnetic behavior and bond order means. - Magnetic behavior: As we know the electron has an electron magnetic dipole moment, which is generally generated by the electron's spin property, which induces an electric charge into motion. As we can see the ...
of the molecular orbitals compared to the original atomic orbitals. • Because the energy of the two electrons is lower than the energy of the individual atoms, the molecule is stable. Figure 9.26: (a) The molecular orbital energy-level diagram for the H2 molecule. (b) The shapes of the molecular orbitals are obtained by squaring the wave
Molecular orbital diagram for f2. A draw the molecular orbital diagram. They also give insight to the bond order of the molecule how many bonds are shared between the two atoms. The relative energies of the sigma orbitals drop below that of the pi orbitals. The molecular orbital theory mo has been introduced for the diatomic hydrogen molecules.
Molecular Orbital Diagram For F2 - A Mechanistic Study Graphene Based Nonvolatile Reram Devices. molecular orbitals of diatomic molecules molecular orbitals of li 2 be 2 to f 2 skills to develop explain how the energy levels of atomic orbitals vary for h li be b c n and o draw relative energy levels diagrams for homonuclear diatomic molecules ...
This problem has been solved! See the answer. Complete the molecular orbital diagram of F2 and F2-. What type of orbital contains the highest energy electron (s) in F2? pi, antibonding. sigma, bonding. sigma, antibonding. pi, bonding. Which atom is larger in size (radius), Cr or Cr 3+?
This video is about MO Diagram #2 - F2
When two fluorine atoms bond, the sigma(2p) bonding molecular orbitals are lower in energy than the pi(2p) bonding orbitals.F2(2+) has a bond order of 2, so ...
Answer (1 of 6): Here is the solution, > * For O2 molecule, > * For F2 molecule, Thanks for reading.
Here is a video that discusses over the Molecular Orbital Diagram for F2+ and F2+. Then compare their bond length, strength, bond order etc. And explaining a...
Problem: Use the molecular orbital diagram shown to determine which of the following is most stable.a. F22+b. Ne22+c. F22-d. O22+e. F2
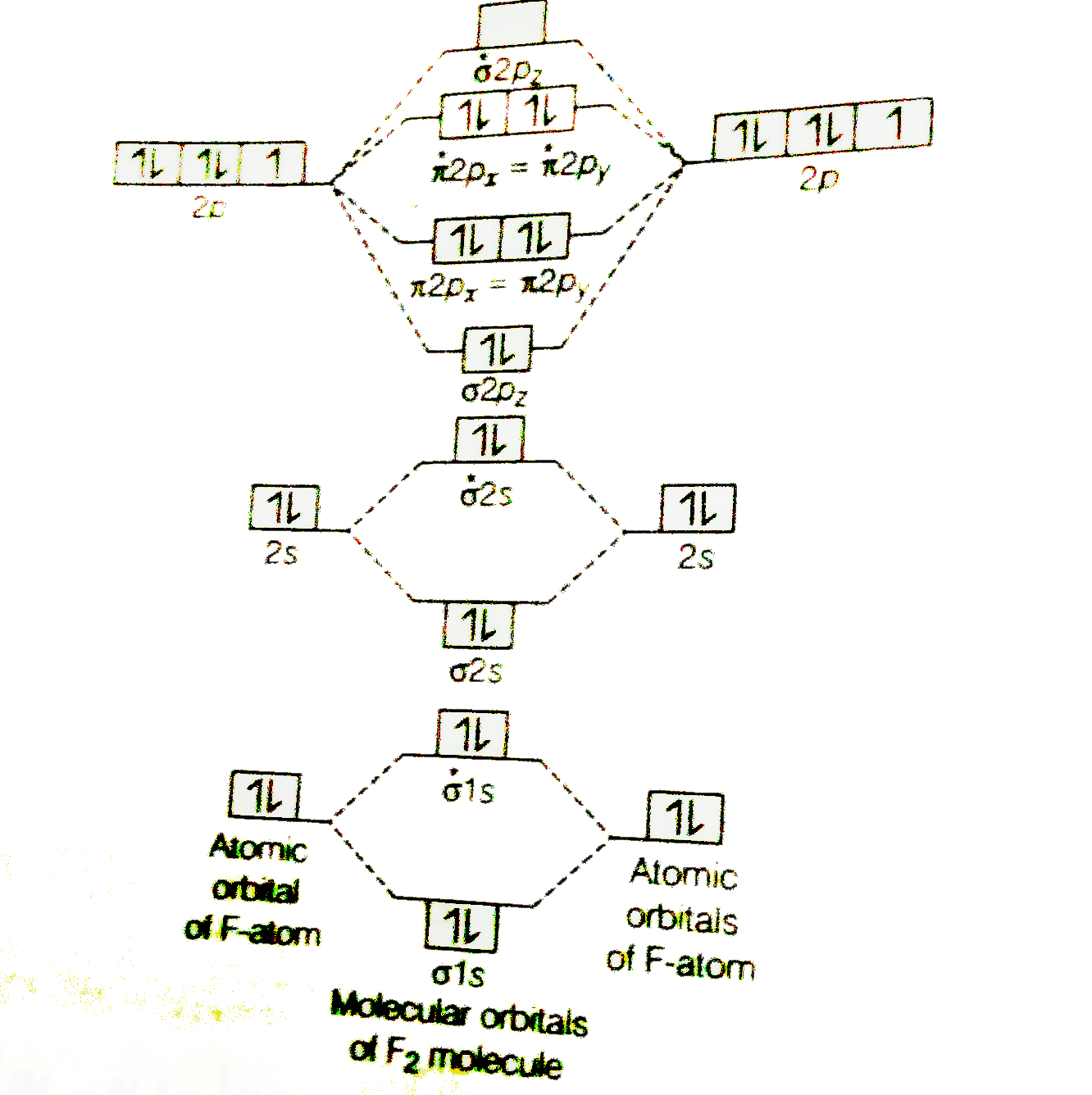
Solved Question 11 A Complete The Molecular Orbital Diagram For F2 Do Not Include The Inner Shell Electrons B Count The Number Of Valence Electrons In F2 C What Is The Bond Order
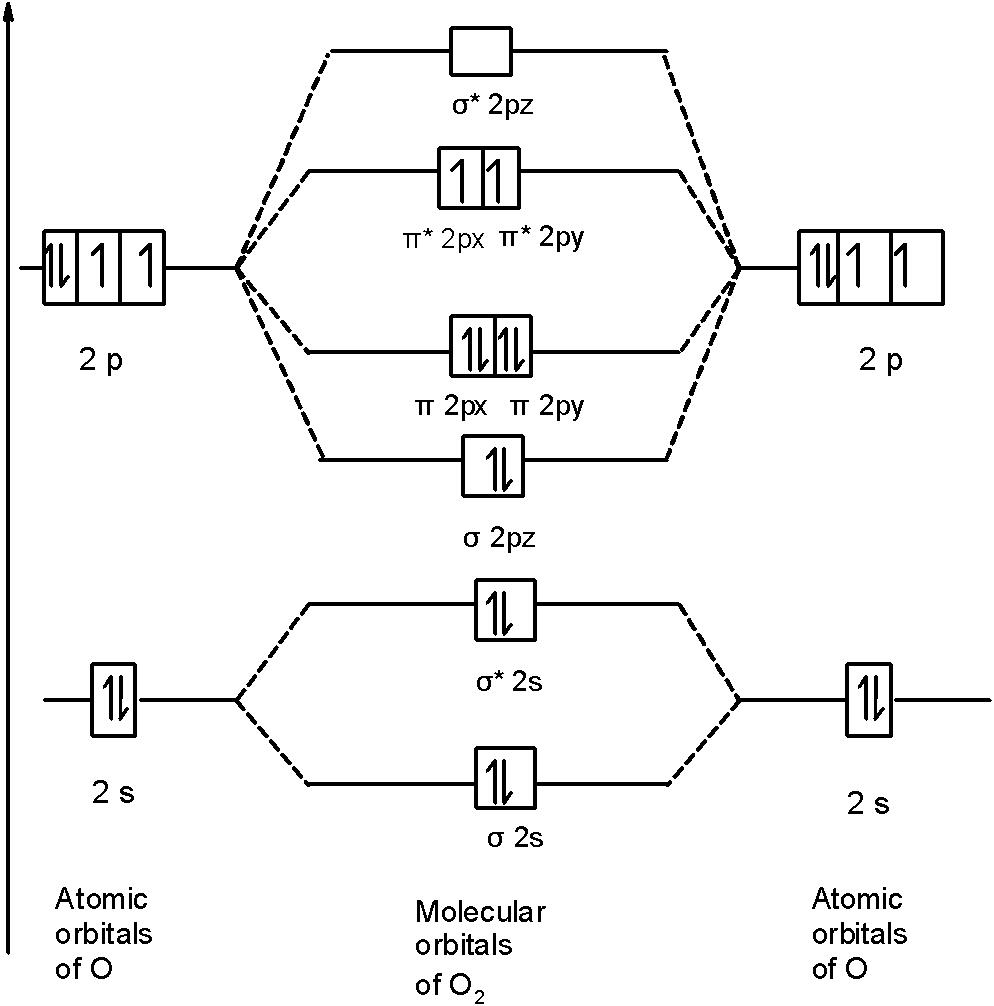
The molecular orbital diagram for an o 2 molecule would therefore ignore the 1s electrons on both oxygen atoms and concentrate on the interactions between the 2s and 2p valence orbitals. F2 molecular orbital diagram. Atomic oxygen stock alamy. Organic chemistry lone pairs bonding pi molecular orbitals. F2 molecular orbital theory.
Orbital Molekul F2. Karena urutan orbital agak berbeda di O 2 dan F 2, yakni orbital 2σ g lebih rendah dari 1π u, orbital molekul untuk O 2, diilustrasikan di Gambar 2.4. Elektron ke-11 dan 12 akan mengisi orbital 1π g yang terdegenerasi dalam keadaan dasar dan spinnya paralel sesuai aturan Hund dan oleh karena itu oksigen memiliki dua ...
7. Draw the molecular orbital diagrams for NO, NO, and NO. For each molecule, determine the bond order, if the molecule is stable, and if the molecule is stable if it is paramagnetic or diamagnetic. Rank the molecules in increasing order of bond strength. Question: 7. Draw the molecular orbital diagrams for NO, NO, and NO.
Solved question 1 by drawing molecular orbital diagrams solved look at the mo diagrams of corresponding neutral diatom when doing molecular orbitals the pi bonds come before sigma for b2 what is the energy level diagram of n2 and f2 brainly in. Information from the mo diagram justify o2s stability and show that its bonding order is 2.
A) F2; B) F2^2+ C) Ne2^2+ D) O2^2+ E) F2^2-2) Use molecular orbital diagrams to determine which of the following are paramagnetic. A) O2^2-B) Ne2^2+ C) O2^2+ D) F2^2+ E) None of the above are paramagnetic; 3) Draw the molecular orbital diagram needed, and determine which of the following is paramagnetic.
Molecular ion (He2)+ has a bond order of 0.5 , while (H2)+ has a bond order 0.5. He2+ is the more stable of the pair because it has two electrons that it can release to form the ion. Is F2 or F2+ more stable? Between F2, F2+, and F2_ which do you think would have the highest bond order, strongest bond, longest bond length.
Before we can draw a molecular orbital diagram for B₂, we must find the in-phase and out-of-phase overlap combinations for boron's atomic orbitals. Then we rank them in order of increasing energy. We can ignore the 1s orbitals, because they do not contain the valence electrons. Each boron atom has one 2s and three 2p valence orbitals. The 2s orbitals will overlap to form 2sσ and 2sσ ...
Molecular Orbital Energy Diagram For F2 Energy Etfs How To Draw Molecular Orbital Diagram 142452 Paramagnetism And What Is The Molecular Orbital Diagram Of O2 And F2 Quora Mo Diagram 2 F2 Youtube Molecular Orbital Theory C2 Molecule Doubly Or Quadruply Bonded Mapping Ignorance Quiz 3 Molecular Orbitals Che 331 Molecular Science Ii Studocu ...
F2 Bonding 35 Images Solved Use The Molecular Orbital Energy Diagram Below To Explain The Formation Of F2 Molecule On The Basis Of Ppt
Use The Molecular Orbital Energy Level Diagram To Show That N 2 Would Be Expected To Have A Triple Bond F 2 A Single Bond And Ne 2 No Bond













0 Response to "36 f2 molecular orbital diagram"
Post a Comment